
Racial and gender disparities in the incidence of anal cancer: analysis of the Nationwide Inpatient Sample (NIS)
Introduction
Anal cancer (AC) is an uncommon form of cancer with a low prevalence of 0.2%, and a lifetime risk of about 1 in 500 people (1,2). AC accounts for 1.5% of gastrointestinal cancer cases, and 4% of cancer-related deaths (3). Symptoms for AC include: rectal bleeding, rectal itching, lump or mass at the anal opening, pain or feeling of fullness in the anal area, changes in bowel movements, abnormal discharge from the anus, and swollen lymph nodes in the anal or groin areas (3,4).
Incidence of AC has risen over the past 25 years with an estimated 8,580 new cases and about 1,600 projected deaths due to AC in 2018 (5). Cultural changes have introduced risk factors contributing to the increase in AC. These changes include infection with human papilloma virus (HPV), suppressed immune system due to human immunodeficiency virus (HIV) or organ transplants, smoking, genital or anal warts, anoreceptive intercourse, multiple sex partners, and history of cervical, vulvar, or vaginal cancer (6-8). Clinical research in AC describes its relationship with income levels and demographic factors, where lower income levels have more severe cases of AC (9-11). Interestingly, other research suggests that higher levels of education are associated with increased incidence in AC for males and females (12).
Delay in diagnosis is not uncommon as patients and physicians might attribute symptoms to hemorrhoids or other colorectal complications. In addition, approximately 20% of patients show no symptoms at time of diagnosis (4). Early detection of AC is easily treatable, but complications arise as AC progresses (13). Current treatments for AC involve combinations of chemoradiation and surgery, which ultimately depends on the staging of AC and response to treatment (4,14).
Racial and gender disparities have been well-documented for most colorectal cancer cases, but AC is yet to be well established. Previous studies suggest that Black males and White females have increased risk for AC (15).
The aim of this study is to assess racial and gender disparities among patients with AC. Greater understanding of AC will result in earlier detection, prevention methods, improved treatments, and better prognosis.
Methods
Data source
This is a 1-year (January – December 2011) retrospective cohort analysis of the National Inpatient Sample (NIS) database which is maintained by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project (HCUP). The NIS database is the largest all-payer in-patient care database publicly available in the United States. It covers 95% of the US population and includes comprehensive abstracted discharged data. The data in the NIS are derived from a stratified sample of 20% of the discharges from all community hospitals (non-federal, short-term, general, and specialty hospitals) in the US (10). The data are weighted back to help make population estimates of the various parameters. For the year 2009, the NIS contains information for 7.4 million weighted discharges from 4,121 hospitals across 44 states (10).
For our study, the use of NIS database was conformed to the data-use agreement from HCUP. This study was reviewed by the University of Arizona, Institutional Review Board and was determined to be exempt from the need for approval.
Patient population
Patients age >18 with diagnosis of AC were identified using the International Classification of Diseases and Injuries codes (Ninth Revision). Patients with missing information of age, diagnosis, and race description were excluded.
Data points collected
From the 2011 NIS database, we retrieved age, gender, race, and diagnoses for AC.
Statistical analysis
Patients were then stratified by race (Whites, Blacks, Hispanics, Asians/Pacific Islanders, and Native Americans). Data are reported as mean ± standard deviation (SD) for continuous descriptive variables, median [range] for ordinal descriptive variables and as proportions for categorical variables. We performed One-way Analysis of Variance (ANOVA), Pearson’s Chi-square test (categorical variables), and independent t-test (continuous variables) to compare demographics. Log-binomial regression was performed to derive relative risk (RR), while adjusting for age and gender, and then for IBD. For our study, we considered P value ≤0.05 as statistically significant. All statistical analyses were performed using Statistical Package for Social Sciences (SPSS, Version 20; SPSS, Inc., Chicago, IL, USA).
Results
There were a total of 6,013,105 cases analyzed in our study, of which 40.5% were male. Of these, 70.7% [4,250,073] of our total cases were classified as White, 15.7% [944,957] as Black, 10.9% [652,905] as Hispanic, 2.2% [130,472] as Asian/Pacific Islander, and 0.6% [34,698] as Native American (Table 1). Of all the patients sampled, 0.02% [1,305] had a diagnosis of AC. Stratifying by race, we see that out of the Whites, Blacks, Hispanics, Asian/Pacific Islanders, and Native Americans, 0.02% [986], 0.02% [222], 0.01% [77], 0.01% [15], and 0.01% [5] had a diagnosis of AC, respectively.
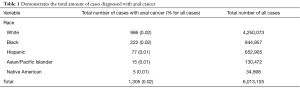
Full table
After adjusting for age, gender, and race in log-binomial regression, we find that out of all patients, increasing age (RR: 1.005, P<0.001) and female gender (RR: 1.14, P=0.02) conferred an increased risk for developing AC, whereas Asian race (RR: 0.51, P=0.01), and Hispanic race (RR: 0.54, P<0.0001) conferred a decreased risk for developing AC (Table 2).
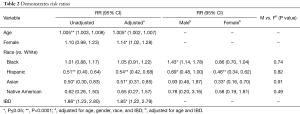
Full table
When taking both race and gender into consideration, we find that Black males (RR: 1.43, P<0.01) have an increased risk of developing AC with no difference seen in Black females (P=0.12). In Hispanics, we see that both Hispanic males (RR: 0.69, P=0.05) and females (RR: 0.46, P<0.0001) had a decreased risk of developing AC. Finally, we see that Asian females (but not Asian males) have a much lower risk of developing AC (RR: 0.33, P<0.01).
Interestingly we did a subgroup analysis of patients with AC and IBD. Patients with diagnosis of IBD had an increased the risk of developing AC (RR: 1.85, P<0.01).
Discussion
Our study sought to identify any racial and gender disparities present in individuals with AC. Our results showed that there are clearly racial disparities in the incidence of AC. Black males have an increased risk of developing AC. Increased risk of AC is less about race and affected more by gender.
Previous studies have shown that the decreased risk of AC seen in Hispanics and Asians may be due to more access to HPV vaccines at early ages (16). Access to HPV vaccines at an early age decreases the likelihood of being infected with any strains associated with AC (11,12,17).
Interestingly, we also saw that patients with IBD have an increased risk for AC. This may be due to Crohn’s disease causing fistula-associated anal carcinoma for patients who suffer from long-term fistulas. Mucin-producing variants have also been reported to cause adenocarcinoma in the anus (18,19). Previous studies have shown that IBD increases an individual’s chance of developing colon cancer which may be true for AC (20). Our data set did not distinguish between Crohn’s or ulcerative colitis and AC. Further studies would need to be done in order to thoroughly understand the relationship of IBD and AC.
This study has limitations. The fact that it is a retrospective student confers inherent limitation. Our sample size encompassed more than 6 million individuals, but due to the small number of individuals with AC (Asian/Pacific Islanders and Native Americans), it may be prudent to perform meta-analyses to generate more power for statistical analysis. Another limitation is that we were not able to separate Crohn’s diseases from ulcerative colitis to better understand which confers more risk of developing AC. One of the risk factors for developing AC is infection with HPV, but this data was not available in the database and could not be included in our analyses. A previous study demonstrates that higher levels of education are associated with increased risk of AC, but in our study, we could not control for levels of education due to our database not having that information (12). Our results show that Black males have increased risk of AC, but we cannot conclude that it is due to race instead of levels of education since we could not control for that variable. Despite the study’s limitations, it is significant enough to show that there are racial and gender disparities in individuals with AC.
Future studies to incorporate larger and more detailed data sets should shed more light on this rare but significant disease. Greater understanding will help identify at-risk populations and eventually lead to improved preventative measures to ultimately reduce the incidence of AC.
Conclusions
Racial disparities and gender differences exist in the incidence of AC. Potential causes for this disparity are disparate access to healthcare, lack of education, and lack of awareness. Greater understanding of the racial disparity in AC can help identify at risk population and eventually lead to improved preventative measures to ultimately reduce the incidence of AC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed by the University of Arizona, Institutional Review Board and was determined to be exempt from the need for approval.
References
- Sasieni PD, Shelton J, Ormiston-Smith N, et al. What is the lifetime risk of developing cancer?: the effect of adjusting for multiple primaries. Br J Cancer 2011;105:460-5. [Crossref] [PubMed]
- Leeds IL, Fang SH. Anal cancer and intraepithelial neoplasia screening: A review. World J Gastrointest Surg 2016;8:41-51. [Crossref] [PubMed]
- Salati SA, Al Kadi A. Anal cancer - a review. Int J Health Sci (Qassim) 2012;6:206-30. [Crossref] [PubMed]
- Uronis HE, Bendell JC. Anal cancer: an overview. Oncologist 2007;12:524-34. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Combes JD, Heard I, Poizot-Martin I, et al. Prevalence and Risk Factors for Anal Human Papillomavirus Infection in Human Immunodeficiency Virus-Positive Men Who Have Sex with Men. J Infect Dis 2018;217:1535-43. [Crossref] [PubMed]
- Beachler DC, Pinto LA, Kemp TJ, et al. An examination of HPV16 natural immunity in men who have sex with men (MSM) in the HPV in Men (HIM) study. Cancer Epidemiol Biomarkers Prev 2018;27:496-502. [Crossref] [PubMed]
- de Martel C, Shiels MS, Franceschi S, et al. Cancers attributable to infections among adults with HIV in the United States. AIDS 2015;29:2173-81. [Crossref] [PubMed]
- Celie KB, Jackson C, Agrawal S, et al. Socioeconomic and gender disparities in anal cancer diagnosis and treatment. Surg Oncol 2017;26:212-7. [Crossref] [PubMed]
- Lin D, Gold HT, Schreiber D, et al. Impact of socioeconomic status on survival for patients with anal cancer. Cancer 2018;124:1791-7. [Crossref] [PubMed]
- Bitterman DS, Grew D, Gu P, et al. Comparison of anal cancer outcomes in public and private hospital patients treated at a single radiation oncology center. J Gastrointest Oncol 2015;6:524-33. [PubMed]
- Benard VB, Johnson CJ, Thompson TD, et al. Examining the association between socioeconomic status and potential human papillomavirus-associated cancers. Cancer 2008;113:2910-8. [Crossref] [PubMed]
- PDQ Adult Treatment Editorial Board PATE. Anal Cancer Treatment (PDQ®): Health Professional Version. National Cancer Institute (US); 2002-2018 Feb 1. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26389221, accessed February 27, 2018.
- Boman BM, Moertel CG, O’Connell MJ, et al. Carcinoma of the anal canal, a clinical and pathologic study of 188 cases. Cancer 1984;54:114-25. [Crossref] [PubMed]
- Noone AM, Howlader N, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014. SEER website. Available online: https://seer.cancer.gov/csr/1975_2014/, Published 2018.
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV Vaccine against Anal HPV Infection and Anal Intraepithelial Neoplasia. N Engl J Med 2011;365:1576-85. [Crossref] [PubMed]
- Apaydin KZ, Fontenot HB, Shtasel DL, et al. Primary Care Provider Practices and Perceptions Regarding HPV Vaccination and Anal Cancer Screening at a Boston Community Health Center. J Community Health 2018;43:792-801. [Crossref] [PubMed]
- Chaparro M, Zanotti C, Burgueño P, et al. Health Care Costs of Complex Perianal Fistula in Crohn’s Disease. Dig Dis Sci 2013;58:3400-6. [Crossref] [PubMed]
- Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, et al. Management of complex perianal Crohn’s disease. Ann Gastroenterol 2017;30:33-44. [PubMed]
- Fornaro R, Frascio M, Denegri A, et al. Chron’s disease and cancer. Ann Ital Chir 2009;80:119-25. [PubMed]


