
Prolonged tumor response associated with sequential immune checkpoint inhibitor combination treatment and regorafenib in a patient with advanced pretreated hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver malignancy and the second most common cause of cancer death worldwide. On a global scale, liver cancer accounts for more than 850,000 new cancer cases annually, and approximately 90% of these are HCC (1). Chronic infection with hepatitis C virus (HCV) or hepatitis B virus (HBV) is the leading cause of HCC (2). The incidence of HCC is rapidly rising in Japan, Europe, and North America due to increased incidence of HCV infection and non-alcoholic fatty liver disease, and in Africa and Middle East due to HBV infection (3,4). HCC is often diagnosed at advanced stages of disease for which highly effective therapies are lacking. At present, sorafenib, a small-molecule multi-kinase inhibitor, is one of the few evidence-based systemic treatment options for patients with advanced HCC (1). In previously untreated patients with advanced disease, the median overall survival was 10.7 months in those treated with sorafenib and 7.9 months in those who received placebo [hazard ratio (HR) =0.69, P<0.001] (5). Another multi-kinase inhibitor, lenvatinib, has been shown to be non-inferior with regards to OS compared to sorafenib when given in first treatment line (6). The multi-kinase inhibitor regorafenib has been reported to provide an overall survival benefit compared with placebo as second line treatment (10.6 vs. 7.8 months; HR =0.62, P<0.001) (7). In RESORCE, regorafenib exhibited a radiological response rate of 10.6% and 6.6% according to mRECIST and RECIST 1.1, respectively (7).
There is a growing evidence suggesting HCC may be considered an immunogenic tumor, arising in an immunosuppressive environment. The chronic inflammation, viral etiology, and cirrhosis underlying the formation of most HCC tumors highlight an intricate relationship between the immune biology and the development of this neoplasm (8). The liver is constitutively immunosuppressive (8) as it promotes systemic tolerance to foreign antigens (9), which prevents excessive reactions to toxins and antigens draining from the enteric circulation (10). The HCC tumor microenvironment (TME) has immunosuppressive features due to the chronic nature of the disease and to tolerogenic characteristics of the liver. HCC exploits this immune tolerance to initiate and promote HCC carcinogenesis and progression. These characteristics of HCC may steer immunotherapeutic strategies to those that inhibit immune suppressive mechanisms, rather than directly increase immune effector function. The recently published open-label, non-comparative CheckMate-040 study reported a radiological response rate of 20% by RECIST 1.1 in 214 patients with advanced HCC with or without HCV or HBV infection receiving nivolumab 3 mg/kg every 2 weeks in the dose expansion phase (11).
Here, we present an impressive case report of a patient with non-virus-associated, advanced pretreated HCC who showed a prolonged treatment response to third-line regorafenib following prior immune checkpoint inhibitor combination treatment with nivolumab and an experimental anti-GITR monoclonal antibody (BMS-986156).
Case presentation
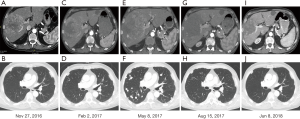
A 68-year-old Caucasian male patient presented with multifocal HCC with multiple bilateral pulmonary metastases in November 2016 after experiencing abdominal unease (Figure 1). Main liver tumor was situated in the right liver lobe with 11×9.7 cm2 and several satellite lesions in liver segments IVa, V and VII (Figure 2A,B). Histological results showed moderately differentiated HCC with trabecular growth pattern and normal neighbouring liver tissue not suggestive of carcinogenesis based on liver cirrhosis. Screening for HBV and HCV was negative; initial AFP was 2,960 µg/L. The patient underwent transarterial embolisation of the main part of the primary liver tumor in the right liver lobe on December 1, 2016. The patient recovered uneventfully and started systemic treatment with sorafenib 400 mg bid on December 16. By this time, his AFP dropped to 382 µg/L as a consequence of tumor embolisation. Despite good tolerability of sorafenib, repeat CT scans on February 22, 2017 showed marked tumor progression in the liver (13×10 cm2 in the right liver lobe) (Figure 2C,D). At this time, AFP had risen to 6,510 µg/L. The patient was registered for a phase 1 clinical trial combining the experimental activating anti-GITR (glucocorticoid-induced TNF receptor) monoclonal antibody BMS-986156 with the anti-PD-1 monoclonal antibody nivolumab (EUDRACT 2015-002505-11). The patient started treatment with intravenous BMS-986156 240 mg in combination with intravenous nivolumab 240 mg on March 14, both given at 2-weekly intervals. The patient tolerated treatment well except potentially treatment-associated general pruritus grade 2 with mild skin changes from scratching but without rash. After 4 administrations of BMS-986156 and nivolumab, restaging CT on May 8 showed further tumor progression to 12.7×17.4 cm2 in the right liver lobe (Figure 2E,F). Radiological evaluation revealed hepatic and pulmonary progression, new peritoneal carcinomatosis as well as tumor infiltration of the Vena cava inferior and central right pulmonary artery embolism. By this time, his AFP had risen to 8,320 µg/L. The patient started anticoagulation using initial enoxaparin with subsequent switch to the new oral anticoagulant (NOAC) apixaban at 5 mg od. As a consequence of radiological tumor progression and clinical deterioration, the patient was not considered a good candidate for treatment with BMS-986156 and nivolumab beyond tumor progression. On June 1, the patient was started on the multi-kinase inhibitor regorafenib at a reduced dose of 80 mg od due to an ECOG performance status of 3. On June 8, the patient was hospitalized for tumor-associated abdominal pain and deterioration of his general health status. On the day of his admission, CT restaging confirmed tumor progression (Figure 1). Regorafenib was reduced to 40 mg od for potential tolerability issues. Within the next month, the patient gradually recovered. Restaging CT on August 15 showed marked hepatic (12×12.8 cm2) (Figure 2G,H), pulmonary and peritoneal tumor regression with incomplete resolution of former pulmonary embolism. By this time, AFP had decreased from 9,230 µg/L on June 1 to 267 µg/L on August 15 and further to 18 µg/L by September 14. On June 8, further reduction of tumor volume was documented on CT scans (Figure 2I,J). In September, the patient was clinically asymptomatic and able to go for a several-week intercontinental journey. Regorafenib was continued at 40 mg od.
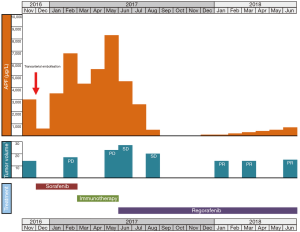
Written informed consent was obtained from the patient for publication of this Case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Discussion
This is an exceptional case of a major tumor response on the multi-kinase inhibitor regorafenib as third line treatment in a patient with advanced, pulmonary metastatic HCC. Our patient was refractory to first-line sorafenib as well as to second-line immune checkpoint inhibitor combination treatment including the anti-PD-1 monoclonal antibody nivolumab and the activating anti-GITR monoclonal antibody BMS-986156 within a clinical trial. Major tumor response included radiological regression of the large primary tumor in the right liver lobe, multiple hepatic satellite lesions and multiple bilateral pulmonary metastases, as well as virtually complete normalization of AFP and substantial, ongoing clinical benefit. Current data do not support a major tumor response to regorafenib monotherapy in patients failing prior sorafenib systemic treatment. As a complicating issue, this patient had impaired tolerability to regorafenib and underwent dose reduction to 40 mg once daily dosing. In the randomized, placebo-controlled and double-blind phase 3 RESORCE study, 10.6% of patients with advanced HCC progressing on sorafenib achieved a radiological response to regorafenib by mRECIST criteria. Although regorafenib is structurally related to sorafenib, the addition of a fluorine atom in the central phenyl ring might result in a higher potency. There are no data as to our knowledge on radiological response rate to regorafenib in patients with sorafenib-refractory disease, but it is suggested to be below 10%. According to the first-line sorafenib registration study (SHARP), 27% of the patients with advanced HCC had disease progression (PD) as their best response, i.e., were sorafenib-refractory. Alternatively, the patient described herein may have experienced a delayed response to combination immunotherapy including nivolumab and BMS-986156. Unlike traditional cytotoxic agents, immunotherapy is known for potentially causing pseudoprogression and/or delayed tumor shrinkage due to the time lag between the disinhibition of the immune response and subsequent antitumor effects. Although these atypical late responses can be seen, they are the exception. In one study of 192 patients treated with pembrolizumab, approximately 10% of patients with progressive disease by irRC at week 12 subsequently achieved some benefit from pembrolizumab therapy as either a response or stable disease (12). Atypical late responses are suggested to be rare in other solid tumors undergoing treatment with anti-PD-(L)1 monoclonal antibodies such as non-small cell lung cancer (NSCLC), kidney or bladder cancer, but concrete data are limited. In the CheckMate-040 clinical trial with nivolumab in patients with advanced HCC, overall radiological response was 20%, and it was 21% in the subgroup of sorafenib progressors (sorafenib pretreated patients) without viral hepatitis (11). Out of the 12 patients with objective radiological responses in the group of sorafenib progressors without viral hepatitis of CheckMate-040, a single patient experienced late treatment response roughly 10 months after starting nivolumab, while all other patients experienced treatment response between roughly 2 and 4 months (11). As a general strategy in immunotherapy, novel second- and third-generation immuno-oncology drugs are currently evaluated to improve response rates and overcome inherent drug resistance. Activating monoclonal antibodies against the glucocorticoid-induced TNF-R-related protein (GITR) (13) is one such approach. One of the first anti-GITR monoclonal antibodies—BMS-986156—has been shown to be well tolerated when added to nivolumab in patients with advanced cancer, with efficacy analysis currently ongoing (14). While there is the potential for increased activity of BMS-986156/nivolumab combination therapy compared to single-agent nivolumab, it is too early to know if late treatment response is a common feature when adding anti-GITR to anti-PD-1 targeted therapy.
Therefore, we may suggest that synergistic activity from sequential immunotherapy and regorafenib is the most plausible mechanisms of action in the present patient. This is supported by the long half-life of the checkpoint inhibitors/monoclonal antibodies of roughly 2–3 weeks, and prolonged pharmacodynamic effects as determined on circulating T-cells (15). Regorafenib is a potent VEGFR2 and VEGFR3 inhibitor, and there is preclinical and clinical data on the synergistic activity of angiogenesis inhibitors and anti-PD-1 targeted therapy. Targeting VEGFR decreased T-regulatory cells and MDSC in a murine liver cancer model (16) and also in colorectal cancer patients (17). Furthermore, VEGF-A produced in the TME of selected solid tumors enhanced the expression of PD-1 involved in CD8+ T cell exhaustion (18). In fact, synergistic activity has further been supported by a clinical trial adding the anti-VEGF monoclonal antibody bevacizumab to nivolumab in patients with advanced kidney cancer (19). Addition of bevacizumab to nivolumab resulted in an improved response rate of 53% (versus 42%) (19). A second phase 2 clinical trial compared the combination of bevacizumab with the anti-PD-L1 monoclonal antibody atezolizumab against the current standard first-line treatment in kidney cancer—sunitinib—and found a substantial improvement of progression-free survival in PD-L1 positive patients (n=164) from 7.8 with sunitinib to 14.7 months with bevacizumab and atezolizumab (HR =0.64; 95% CI, 0.38–1.08; P=0.095) (20). In the same patients, radiological response was improved from 27% to 46% (20). These data suggest strong synergistic effects between PD-(L)1 and VEGF targeting agents. We believe that the present data are supporting combination studies of regorafenib with PD-(L)1 targeted monoclonal antibodies in patients with advanced HCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [Crossref] [PubMed]
- McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 2015;19:223-38. [Crossref] [PubMed]
- Korangy F, Hochst B, Manns MP, et al. Immune responses in hepatocellular carcinoma. Dig Dis 2010;28:150-4. [Crossref] [PubMed]
- Waller LP, Deshpande V, Pyrsopoulos N. Hepatocellular carcinoma: A comprehensive review. World J Hepatol 2015;7:2648-63. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-390. [Crossref] [PubMed]
- Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163-73. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Khan H, Pillarisetty VG, Katz SC. The prognostic value of liver tumor T cell infiltrates. J Surg Res 2014;191:189-95. [Crossref] [PubMed]
- Cantor HM, Dumont AE. Hepatic suppression of sensitization to antigen absorbed into the portal system. Nature 1967;215:744-5. [Crossref] [PubMed]
- Chan T, Wiltrout RH, Weiss JM. Immunotherapeutic modulation of the suppressive liver and tumor microenvironments. Int Immunopharmacol 2011;11:879-89. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Hodi FS, Ribas A, Daud A. Evaluation of immune-related response criteria (irRC) in patients (pts) with advanced melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475. J Clin Oncol 2014;32:abstr 3006.
- Placke T, Kopp HG, Salih HR. Glucocorticoid-induced TNFR-related (GITR) protein and its ligand in antitumor immunity: functional role and therapeutic modulation. Clin Dev Immunol 2010;2010:239083. [Crossref] [PubMed]
- Siu LL, Steeghs N, Meniawy T, et al. Preliminary Results of a Phase 1/2a Study of BMS-986156 (glucocorticoid-induced tumor necrosis factor receptor–related gene [GITR] agonist) Alone and in Combination With Nivolumab in Patients With Advanced Solid Tumors. J Clin Oncol 2017;35:104. [Crossref]
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167-75. [Crossref] [PubMed]
- Cao M, Xu Y, Youn JI, et al. Kinase inhibitor Sorafenib modulates immunosuppressive cell populations in a murine liver cancer model. Lab Invest 2011;91:598-608. [Crossref] [PubMed]
- Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res 2013;73:539-49. [Crossref] [PubMed]
- Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 2015;212:139-48. [Crossref] [PubMed]
- Gao J, Karam JA, Wood CG, et al. Clinical activity, immune and molecular correlates of nivolumab vs. nivolumab plus bevacizumab vs nivolumab plus ipilimumab in metastatic renal cell carcinoma. Cancer Res 2017;77:CT083. [Crossref]
- McDermott DF, Atkins MB, Motzer RJ, et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). J Clin Oncol 2017;35:abstr 431.


