
Low prevalence of deficient mismatch repair (dMMR) protein in locally advanced rectal cancers (LARC) and treatment outcomes
Introduction
Colorectal cancers (CRC) primarily develop via a chromosomal instability pathway, though 12–15% of tumors also develop due to a deficient DNA mismatch repair (dMMR) characterized by microsatellite instability (MSI) (1). Most studies evaluating the incidence of dMMR have looked at CRC collectively, with a major focus on its differential incidence across stages and benefit with 5-fluorouracil (2-4). The available evidence in a pure rectal cancer cohorts suggests a lower incidence of dMMR, approximating 1–3% (4-6). Beyond incidence rates of dMMR status, there is enough evidence to suggest good correlation between measuring MMR status by immunohistochemistry (IHC) for the MMR proteins, and MSI analysis by polymerase chain reaction (PCR) (7,8). IHC has high sensitivity for detecting MSI-H status (≥90%) and is a cost effective method to screen and evaluate MSI status.
Available evidence from India suggests that rectal cancers are commoner than colonic primaries, the age at incidence is younger and there is an advanced stage at presentation as compared to available data from North American and European countries (9-11). Previous data published from our institution (patients treated between June 2006 and December 2010) and other centres in India have shown curative resection ranging from 72% to 98%, though overall survivals (OS) have been similar (12-14) post treatment with long course chemoradiation (LCRT) in LARC. However, all the Indian studies used computerized tomography (CT) based imaging for rectal primary, whereas the current imaging standard for local rectal primary should be MRI based due to its superiority in assessment of primary, mesorectal nodes and extramesorectal nodes (15-17).
Studies from India have commented on the relatively increased bulk of disease and advanced stage of presentation of non-metastatic rectal cancers, but there is unclear evidence in terms of factual documentation and classification of these tumors as bulky or ‘ugly’ tumors. The classification of LARC into favourable, intermediate and advanced as suggested by Blomqvist et al. is a convenient and easy to use method and is an indicator of local disease burden (18).
Methods
Patients with LARC who were offered LCRT, as per institution protocol during the period of 1st January 2014 to 31st December 2015 at the Department of Gastrointestinal Oncology, Tata Memorial Hospital (TMH) in Mumbai were evaluated. The study was approved by the Institutional Review Board (IRB) and Ethics Committee (EC) (IEC/1116/1799/001) and was conducted as per the declaration of Helsinki guidelines. Patient data was extracted from a prospectively maintained rectal cancer database at TMH.
Patients included in the study satisfied all the following criteria:
- Histologically confirmed adenocarcinoma of the rectum, either T3/T4 and or node (N) positive as per clinical examination and contrast enhanced MRI (CE-MRI) of the rectum;
- No evidence of metastases, based on contrast enhanced CT (CECT) scans or 18-FDG contrast enhanced positron emission tomography (PET) scan;
- Planned for LCRT based on staging characteristics;
- Availability of rectal biopsy specimen for dMMR status testing by IHC.
Baseline staging for all patients included a complete physical examination, colonoscopy, CECT (Thorax, Abdomen) or 18 FDG PET-CT, CE-MRI pelvis and carcinoembryonic antigen (CEA) levels.
IHC for MMR status
In all the cases, histopathologic sections, including hematoxylin and eosin (H&E) stained and IHC stained sections were reviewed by in house pathologists.
Patients satisfying the above criteria were included in the study and the formalin fixed paraffin embedded blocks (FFPE) of these patients were retrieved and tested for MMR status by IHC for the protein expressed by the MMR genes, MLH1, MSH2, MSH6, and PMS2, respectively. IHC staining was performed using the MACH2 Universal HRP Polymer detection kit (Biocare Medical, CA, USA) including peroxidase/3-3-diaminobenzidine tetrahydrochloride (DAB). Details of the various IHC antibody markers, have been enlisted in Table S1. IHC scoring for the following markers were as follows:

Full table
MLH1, MSH2, MSH6 or PMS2
- Tumor cells nuclei exhibiting brown staining for each of these markers is considered as MMR proficient (pMMR);
- Tumor cell nuclei not displaying any brown staining for any of these markers is considered as MMR deficient (dMMR).
Normal mucosa and infiltrating leucocytes were used as internal controls for evaluation of the IHC.
Radiological classification of tumors
Reports of baseline CE-MRI’s of patients were reviewed and all tumors were retrospectively classified as into three groups as detailed below:
- Favorable—tumors satisfying all the following criteria:
- T3 tumors;
- circumferential margin (CRM) negative;
- node negative;
- Intermediate:
- T3 tumors with CRM threatened, but technically CRM negative or/and N1/N2;
- T4 tumors with peritoneal and vaginal involvement only, irrespective of N status;
- Advanced:
- T4 with overgrowth to prostate, seminal vesicles, base of urinary bladder, pelvic side walls or floor, sacrum, positive lateral lymph nodes;
- All CRM positive tumors.
Treatment protocol
Radiotherapy protocol—radiotherapy was given to a dose of 45–50.4 Gy in conventional fractionation (180–200 cGy per fraction, one fraction per day and five fractions per week) with treatment ranging between 5–5.5 weeks.
Chemotherapy protocol—all the patients received oral capecitabine concurrently to a dose of 850 mg/m2 in twice daily for the duration of radiotherapy. Patients receiving 5 FU as concurrent chemotherapy were not included in study. Interruptions in LCRT ≥1 week, either due to radiotherapy or chemotherapy, were also noted.
Evaluation for resection
Patients were evaluated with CE-MRI 6–8 weeks post completion of LCRT. Responses were recorded as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) based on changes in signal tumor intensity, regression in tumor and nodal size, regression in CRM status and presence of fibrosis on T2 weighted sequences (16,19). Patients were taken up for surgery based on multidisciplinary assessment if they satisfied the following criteria:
- CRM negativity;
- Absence of extension through the greater sciatic notch, encasement of external iliac vessels, para-aortic lymphadenopathy, or sacral invasion above S2–S3 junction;
- R0 resection possible.
Patients with extensive side-wall involvement were considered for local resection based on a case-to-case scenario.
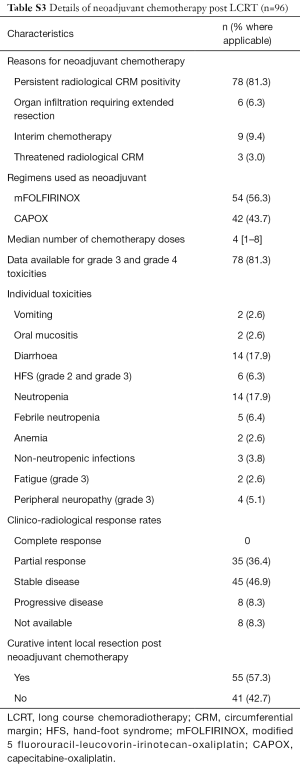
Patients who were considered unresectable post LCRT were offered chemotherapy with an attempt to further downstage disease status to resectable status. Patients were offered potentially neoadjuvant intent capecitabine-oxaliplatin (CAPOX), or modified 5 fluorouracil-leucovorin-irinotecan-oxaliplatin (mFOLFIRINOX without bolus 5 FU) for 2–3 months based on assessment by medical oncologist. Our institution as well as others have previously published data regarding this approach (20,21). Dosages and schedules were as per standard schedules. A repeat MRI was conducted post neoadjuvant therapy to assess resectability status. The reasons for neoadjuvant chemotherapy were documented for all patients who received the same. Patients who were not feasible for resection post neoadjuvant chemotherapy were offered the option of continuing chemotherapy with palliative intent.
Patients were offered CAPOX or single agent capecitabine as adjuvant chemotherapy to complete 6 months of perioperative therapy. Post completion of planned therapy, patients were kept on surveillance as per institution protocol.
Prognostic factors
Pre-defined prognostic factors evaluated for correlation with OS were:
- Younger age at diagnosis (≤40 vs. > 40 years);
- Degree of differentiation (poorly differentiated vs. well/moderately differentiated adenocarcinoma);
- Signet ring (SR) histology (presence vs. absence);
- mucinous histology (presence vs. absence);
- tumor location (upper vs. mid vs. lower);
- T stage;
- N stage;
- Favourable vs. intermediate vs. advanced as per previously mentioned criteria;
- Baseline CEA status [> upper limit of normal (ULN) vs. within ULN].
Treatment related factors and correlation with survival
Pre-defined post LCRT related factors were assessed for correlation with OS:
- ypT0-T2 vs. ypT3-4;
- ypN0 vs. ypN+;
- TRG 1-3 vs. TRG 4-5;
- Margin status (involved vs. uninvolved);
- Presence of pathological CR vs. absence of CR.
Clinical data collection and statistics
For the purposes of this study demographic data and baseline clinical and tumor characteristics, LCRT, surgical procedures and outcomes were collected from the charts maintained prospectively (GI Medical Oncology Information System and electronic medical record system). All data was entered in SPSS software version 21 (IBM) and used for analysis. Descriptive statistics including median, frequency and percentage for categorical variables is used to describe age, gender distribution, treatment and response to treatment. Survival outcomes in terms of recurrence free survival (RFS) and OS were analysed for patients undergoing resection of the primary. Median RFS was calculated from the date of diagnosis to the date of clinical or radiological evidence of disease recurrence. Survival for patients not undergoing resection was reported as event free survival (EFS). Median EFS was calculated from the date of diagnosis to the date of clinical or radiological evidence of disease progression or the last follow-up date. Median OS was calculated from the date of diagnosis until last follow-up or death. Survival analysis was done using Kaplan-Meier estimates and log rank test for bivariate comparisons. Variables achieving statistical significance (P<0.05) on univariate analysis were evaluated for multivariate analysis by cox-regression.
Results
MMR status
A total of 419 patients were evaluated for LARC in TMH in the pre-specified time period, of whom 354 satisfied the stated inclusion criteria and were treated at TMH with LCRT. Of these 354 patients, 296 were assessable for MMR status based on tissue adequacy for testing (272 pre NACTRT and 24 post-operative specimens). Three patients (1.01%; n=296) had dMMR status, while the remaining 293 patients had proficient MMR status. The first patient had loss of MLH1 and PMS2 expression, second patient had loss of MLH1 and MSH 6 expression while the third patient had loss of MSH2 and MSH6 expression.
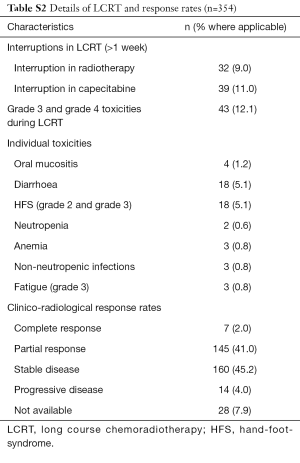
Baseline characteristics and details of baseline T & N staging as per MRI is reported in Table 1. Administration of LCRT and responses along with details including responses to neoadjuvant chemotherapy post LCRT are reported in Tables S2,S3 respectively.

Full table
Full table
Full table
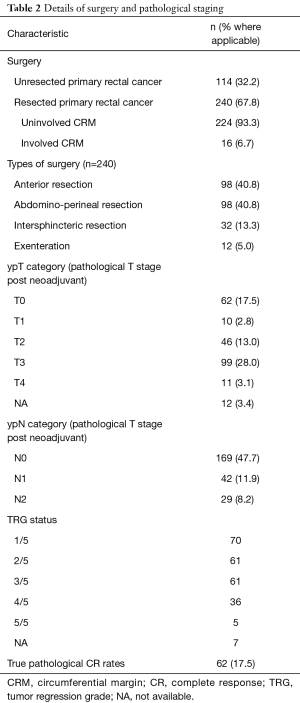
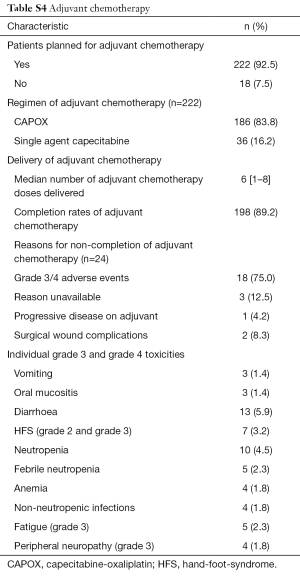
Resection details and pathological staging are described in Table 2. A total of 240 patients (67.8%) underwent curative intent resection of local rectal cancer. The majority of surgeries conducted were anterior resection and abdominal perineal resection in 98 patients (40.8%) each. Pathological CR was seen in 62 patients (17.5%; n=354). Adjuvant chemotherapy details are reported in Table S4.
Full table
Full table
Recurrence patterns and survival
With a median follow-up of 32 months, a total of 146 patients (41.2%) had events (either recurrence or progression) in the entire cohort with median EFS not reached (Table 3). The estimated 3-year RFS for the resected cohort was 63.5%, while the 3-year EFS for the unresected cohort was 15.2%. One hundred ten patients (31.1%) had died at time of follow-up with median OS not achieved. The estimated 3-year OS for the resected cohort was 85.2% while it was 15.8% for the unresected cohort.
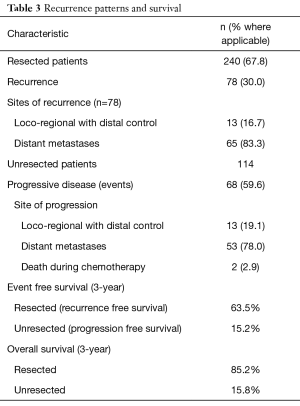
Full table
Prognostic factors for OS—pre-treatment
Of the factors planned for evaluation as prognostic, signet ring histology (P<0.001), mucinous histology (P=0.047), T stage (P=0.030) and the subdivisions of LARC (P=0.045) achieved statistical significance on univariate analysis. On multivariate analysis, only signet ring cancers retained statistical significance in predicting outcomes (P<0.001; 95% CI, 1.27–3.06) (Table 4).
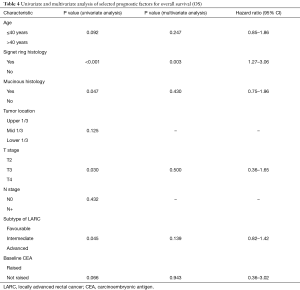
Full table
Predictive factors for OS—post LCRT
Of the factors evaluated as predictive of outcomes post LCRT on pathological staging, ypT0-2 vs. ypT3-4 status (P=0.041), and margin status (P=0.001) predicted for statistically significantly reduced OS. On multivariate analysis, both factors, presence of margin involvement (P=0.010; 95% CI, 1.27–5.70) and higher ypT status predicted for reduced OS (P=0.048; 95% CI, 0.21–0.99).
Delays in LCRT, either radiotherapy or concurrent chemotherapy component of greater than 1 week also predicted for statistically significant reduction in OS (P=0.003) (Table 5).
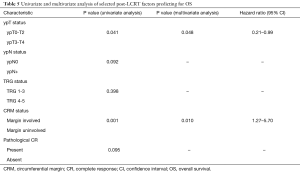
Full table
Discussion
The concept of MSI-H status characterizing an entire subset of CRC along with the development of the CMS molecular subtyping has completely changed our understanding of CRC (22-24). The use of MSI-H status as a tumor agnostic marker for response to Pembrolizumab has further underscored the importance of this biomarker in the clinical setting (25). In such a scenario, the use of a cost-effective and largely interconvertible method of MSI testing (as opposed to PCR), i.e., MMR protein testing by IHC, expands the reach and usefulness of such testing.
The current study has a carefully selected cohort of LARC patients (majorly stage III) receiving a consistent treatment protocol of LCRT. The prevalence of dMMR status seen in this study was 1.01%. This is a reiteration of the low prevalence of dMMR in rectal cancers as opposed to the blanket value of dMMR status of 15% in CRC that is often quoted (3). A similarly low prevalence of dMMR was seen in the stage II rectal cancer cohort in the QUASAR study (1%) (4). Such results suggest that dMMR will not be a major determinant of prognosis or treatment in rectal cancers. Further clinical evidence of such an effect is seen with the relative lack of benefit of adding oxaliplatin with 5-FU based concurrent chemoradiation as neoadjuvant therapy. Besides an increase in pathological CR rates in one study, none of the studies showed a relevant improvement in resection rates or survival (26-28). The low prevalence of dMMR status may circumvent the potential benefit seen with oxaliplatin based regimens in dMMR rectal cancers (29). Additionally, considering the controversial risk (long term toxicities, especially oxaliplatin induced neuropathy) vs. benefit ratio of adjuvant chemotherapy in rectal cancer patients treated with LCRT and surgery, the low prevalence of dMMR lends itself to the hypothesis generation that single agent 5-FU or capecitabine alone as adjuvant chemotherapy may suffice as against an oxaliplatin containing doublet (30,31). Importantly, our study potentially rules out use of anti-dMMR immunotherapy in advanced rectal cancers (25), which in turn emphasizes the need to direct our efforts towards the development of anti-MMR proficient therapies.
The cohort of patients seen in this study had a number of unfavourable characteristics—a young age (36.4% <40 years), a high incidence of signet ring histology (17.5%), and an unfavourable nature of local rectal cancer (43.8%). The division of LARC into subsets as suggested by Blomqvist et al. was used with certain modifications in this study and helps us to effectively show that a high percentage of tumors presenting in our centre have a high local burden of disease and ‘unfavourable’ baseline characteristics. While such a stratification stresses on being predictive for loco-regional failure rates (as opposed to factors such as extramural vascular invasion, EMVI, predicting for systemic recurrences), evidence for poor local control in rectal cancers predisposing to development of distant metastases is known (18,19). This is evident in the nature of recurrences and progression seen in the current study. Patients with resected disease predominantly had distant recurrences as opposed to loco-regional recurrences (83.3% vs. 16.7%). A similar pattern was evinced in patients who were unable to undergo surgery (distant progression as first event 78% vs. 19.1% as loco-regional progression as first event). Such a recurrence pattern is partially explainable on the basis of the molecular and clinical etiology of rectal tumors. As per the CMS classification, most rectal tumors are of the CMS—4 mesenchymal type. These tumors are known to have MSI-S status (as seen in the current study also) and an epithelial-mesenchymal transition (EMT) genotype, present in an advanced stage, and have poorer OS (24). When this is superimposed with the fact that signet ring cancers (17.5% of patients in this study as opposed to the usual 1% seen in other studies) have a tendency to present and recur with distant metastases, we are able to come up with a feasible explanation for the recurrence pattern seen with this study (32,33). Such a clinical and biological profile in the setting of a low incidence of dMMR suggests that future efforts in the neoadjuvant setting for LARC should be directed against pMMR tumors and EMT genotypes and possibly not with the use of MSI-H directed drugs like pembrolizumab (34,35).
Despite the unfavourable characteristics of the disease cohort in this study, 67.8% of patients were able to undergo a curative local resection with the majority being R0 resections (93.3%). This is in keeping with CRM positivity rates seen in most population based non-trial studies across the world (36-38). We have also shown that neoadjuvant chemotherapy post LCRT is a feasible and effective option in an attempt to further downstage tumors to curative intent resection. Fifty-seven point three percent of patients were able to undergo resection post neoadjuvant chemotherapy as opposed to continuing chemotherapy with palliative intent without local resection. Besides down staging, the use of chemotherapy is an effective measure of interim systemic disease control of potential micro metastatic disease and also provides for assessment of tumor biology (20,21). Our continued use of adjuvant chemotherapy (92.5% of patients) post resection is also keeping in line with our philosophy that the majority of tumors in our setting need adequate systemic control of disease and not only local disease control.
The EFS and OS seen in this study, within the confines of a short follow-up with respect to rectal cancers, appear encouraging. The standout prognostic feature appears to be the presence of signet ring histology contributing to poorer outcomes in terms of OS as well as EFS (not reported). The biological and clinical differences between signet ring and non-signet ring CRC is well known in terms of outcomes and presentation (32,33,39). ypT stage post LCRT also achieved statistical significance on multivariate analysis (P=0.05) reiterating advanced nature of disease on presentation and indicating urgent need to develop strategies to downstage MMR proficient, signet ring disease in a better way.
We also identified a delay or interruption (>1 week) in chemoradiation as a predictor of inferior OS. As per our knowledge, this is the first time such a variable has been identified in rectal cancers as predictive. While treatment related delays in LCRT are inevitable if related to complications (e.g., severe local skin reactions, large volume tumor bleed, severe diarrhoea, HFS, etc.), it is important to realise that various tumor dynamics are at play from the initiation of treatment for LARC to potential surgery (approximately 3–4 months).
Multiple limitations in this study have to be acknowledged. The data generated is retrospective and from a single tertiary cancer referral centre where the possibility of a referral bias in terms of more advanced LARC is likely. We do not have PCR sequencing as a comparator or complementary method to reconfirm the findings of MSI status as diagnosed by IHC. The method of diagnosis of signet ring histology is based on qualitative as opposed to a quantitative description in terms of percentages. The classification of LARC into different categories does not have information on factors such as EMVI, which is an important predictor of systemic relapse. The duration of follow-up in our study is 32 months, which is relatively short in the context of rectal cancers being potentially treated with curative intent.
In conclusion, we have shown that assessment of MMR status by IHC is feasible in a large LARC cohort and a majority of these tumors have proficient MMR status. This suggests that MSI as a biomarker may have limited applicability in the management of rectal cancers per se, unless we develop immunotherapeutic agents directed against pMMR tumors. A higher than usual prevalence of signet ring rectal cancers was seen in this study and signet ring histology was a powerful predictor of inferior outcomes. Neoadjuvant chemotherapy LARC to downstage the tumor further post NACTRT, is also an important aspect of our study. We also identified, possibly for the first time, that interruptions in LCRT beyond 1 week predicted for inferior OS.
Acknowledgements
We thank Tata Memorial Center Research Administrative Council (TRAC) for providing financial support for this study and Cadila Healthcare Private Limited supported MMR testing through educational grants to Tata Memorial Center, Parel, Mumbai.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board (IRB) and Ethics Committee (EC) (IEC/1116/1799/001) and was conducted as per the declaration of Helsinki guidelines.
References
- Boland CR, Goel A. Microsatellite Instability in Colorectal Cancer. Gastroenterology 2010;138:2073-87.e3. [Crossref] [PubMed]
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor Microsatellite-Instability Status as a Predictor of Benefit from Fluorouracil-Based Adjuvant Chemotherapy for Colon Cancer. N Engl J Med 2003;349:247-57. [Crossref] [PubMed]
- Sargent DJ, Marsoni S, Monges G, et al. Defective Mismatch Repair As a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J Clin Oncol 2010;28:3219-26. [Crossref] [PubMed]
- Hutchins G, Southward K, Handley K, et al. Value of Mismatch Repair, KRAS, and BRAF Mutations in Predicting Recurrence and Benefits From Chemotherapy in Colorectal Cancer. J Clin Oncol 2011;29:1261-70. [Crossref] [PubMed]
- The clinical features of rectal cancers with high-frequency microsatellite instability (MSI-H) in Japanese males. Cancer Lett 2004;216:55-62. [Crossref] [PubMed]
- Samowitz WS, Curtin K, Wolff RK, et al. Microsatellite instability and survival in rectal cancer. Cancer Causes Control CCC 2009;20:1763-8. [Crossref] [PubMed]
- Kawakami H, Zaanan A, Sinicrope FA. MSI testing and its role in the management of colorectal cancer. Curr Treat Options Oncol 2015;16:30. [Crossref] [PubMed]
- Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol 2017;12:24. [Crossref] [PubMed]
- Gupta S, Bhattacharya D, Acharya AN, et al. Colorectal carcinoma in young adults: a retrospective study on Indian patients: 2000-2008. Colorectal Dis 2010;12:e182-9. [Crossref] [PubMed]
- Mohandas KM. Colorectal cancer in India: controversies, enigmas and primary prevention. Indian J Gastroenterol 2011;30:3-6. [Crossref] [PubMed]
- Patil PS, Saklani A, Gambhire P, et al. Colorectal Cancer in India: An Audit from a Tertiary Center in a Low Prevalence Area. Indian J Surg Oncol 2017;8:484-90. [Crossref] [PubMed]
- Engineer R, Basu T, Chopra S, et al. Factors influencing response to neoadjuvant chemoradiation and outcomes in rectal cancer patients: tertiary Indian cancer hospital experience. J Gastrointest Oncol 2015;6:155-64. [PubMed]
- Kunheri B, Gurram B, Madhavan R, et al. Preoperative long-course chemoradiation for localized rectal cancer: A retrospective comparison of response and outcome between 5-fluorouracil/leucovorin versus capecitabine. Indian J Cancer 2016;53:518. [Crossref] [PubMed]
- Bansal V, Bhutani R, Doval D, et al. Neo adjuvant chemo-radiotherapy and rectal cancer: can India follow the West? J Cancer Res Ther 2012;8:209-14. [Crossref] [PubMed]
- Mathur P, Smith JJ, Ramsey C, et al. Comparison of CT and MRI in the pre-operative staging of rectal adenocarcinoma and prediction of circumferential resection margin involvement by MRI. Colorectal Dis 2003;5:396-401. [Crossref] [PubMed]
- Arya S, Das D, Engineer R, et al. Imaging in rectal cancer with emphasis on local staging with MRI. Indian J Radiol Imaging 2015;25:148. [Crossref] [PubMed]
- Jhaveri KS, Hosseini-Nik H. MRI of Rectal Cancer: An Overview and Update on Recent Advances. AJR Am J Roentgenol 2015;205:W42-55. [Crossref] [PubMed]
- Blomqvist L, Glimelius B. The “good”, the “bad”, and the “ugly” rectal cancers. Acta Oncol 2008;47:5-8. [Crossref] [PubMed]
- Patel UB, Blomqvist LK, Taylor F, et al. MRI After Treatment of Locally Advanced Rectal Cancer: How to Report Tumor Response—The MERCURY Experience. AJR Am J Roentgenol 2012;199:W486-95. [Crossref] [PubMed]
- Ostwal V, Engineer R, Ramaswamy A, et al. Surgical outcomes of post chemoradiotherapy unresectable locally advanced rectal cancers improve with interim chemotherapy, is FOLFIRINOX better than CAPOX? J Gastrointest Oncol 2016;7:958-67. [Crossref] [PubMed]
- Sclafani F, Brown G, Cunningham D, et al. Systemic Chemotherapy as Salvage Treatment for Locally Advanced Rectal Cancer Patients Who Fail to Respond to Standard Neoadjuvant Chemoradiotherapy. The Oncologist 2017;22:728-36. [Crossref] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Sveen A, Bruun J, Eide PW, et al. Colorectal Cancer Consensus Molecular Subtypes Translated to Preclinical Models Uncover Potentially Targetable Cancer Cell Dependencies. Clin Cancer Res 2018;24:794-806. [Crossref] [PubMed]
- Guinney J, Dienstmann R, Wang X, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350-6. [Crossref] [PubMed]
- Diaz LA, Marabelle A, Delord JP, et al. Pembrolizumab therapy for microsatellite instability high (MSI-H) colorectal cancer (CRC) and non-CRC. J Clin Oncol 2017;35:3071. [Crossref]
- Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011;29:2773-80. [Crossref] [PubMed]
- Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 2012;30:4558-65. [Crossref] [PubMed]
- Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol 2012;13:679-87. [Crossref] [PubMed]
- Tougeron D, Mouillet G, Trouilloud I, et al. Efficacy of Adjuvant Chemotherapy in Colon Cancer With Microsatellite Instability: A Large Multicenter AGEO Study. J Natl Cancer Inst 2016.108. [PubMed]
- Petrelli F, Coinu A, Lonati V, et al. A systematic review and meta-analysis of adjuvant chemotherapy after neoadjuvant treatment and surgery for rectal cancer. Int J Colorectal Dis 2015;30:447-57. [Crossref] [PubMed]
- Breugom AJ, Swets M, Bosset JF, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015;16:200-7. [Crossref] [PubMed]
- Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol 2012;19:2814-21. [Crossref] [PubMed]
- Inamura K, Yamauchi M, Nishihara R, et al. Prognostic Significance and Molecular Features of Signet-Ring Cell and Mucinous Components in Colorectal Carcinoma. Ann Surg Oncol 2015;22:1226-35. [Crossref] [PubMed]
- Vu T, Datta PK. Regulation of EMT in Colorectal Cancer: A Culprit in Metastasis. Cancers 2017.9. [PubMed]
- Trial of Chemoradiation and Pembrolizumab in Patients With Rectal Cancer - Full Text View - ClinicalTrials.gov. [cited 2018 Mar 1]. Available online: https://clinicaltrials.gov/ct2/show/NCT02586610
- Butler EN, Chawla N, Lund J, et al. Patterns of colorectal cancer care in the United States and Canada: a systematic review. J Natl Cancer Inst Monogr 2013;2013:13-35. [Crossref] [PubMed]
- De Caria K, Rahal R, Niu J, et al. Rectal cancer resection and circumferential margin rates in Canada: a population-based study. Curr Oncol 2015;22:60-3. [Crossref] [PubMed]
- Rickles AS, Dietz DW, Chang GJ, et al. High Rate of Positive Circumferential Resection Margins Following Rectal Cancer Surgery: A Call to Action. Ann Surg 2015;262:891-8. [Crossref] [PubMed]
- Tajiri K, Sudou T, Fujita F, et al. Clinicopathological and Corresponding Genetic Features of Colorectal Signet Ring Cell Carcinoma. Anticancer Res 2017;37:3817-23. [PubMed]


