Lower lymph node yield following neoadjuvant therapy for rectal cancer has no clinical significance
Introduction
A minimum yield of 12 lymph nodes following resection of colorectal cancer is often “mandatory” for accurate nodal staging as lymph node status has been shown to be a major prognostic factor for oncological outcomes (1). Interestingly, neoadjuvant chemoradiotherapy administered for locally advanced rectal cancer has however been associated with a considerable reduction in the lymph node yield following resection (2,3).
The implications of this lower lymph node yield continue to be debated in the literature. There are authors who have suggested that efforts should be made to ensure that a minimum of 12 lymph nodes are retrieved from the resection specimen to maintain the validity of node negative disease (4,5), while other authors have proposed that a reduction in lymph node yield implies better prognosis for the patient and that this minimum number of 12 lymph nodes is not necessary. Gurawalia et al. showed that pathological complete response (PCR) rates were significantly higher in patients with less than 12 lymph nodes harvested compared with those who had at least 12 (40% vs. 26%, P<0.05) (6). Similarly, disease-free survival (DFS) has been shown to be better in patients who yielded less than 12 lymph nodes (7).
We therefore performed this study to determine if harvesting a minimum of 12 lymph nodes after surgical resection for rectal cancer following neoadjuvant chemoradiotherapy affected oncologic outcomes.
Methods
A retrospective study of all patients who were treated for rectal cancer in our institution from January 2008 to December 2014 was performed. Rectal cancer was defined as adenocarcinomas located within 15 cm from the anal verge (5). All patients were staged according to the 6th or 7th editions of the AJCC manual for rectal cancer, depending on the most recent edition present at time of diagnosis (8). Patients were excluded if they had metastatic disease (M1), or had a history of familial colorectal cancer syndromes. It is our institutional practice to discuss all new cases of rectal cancer in our multidisciplinary tumour board meeting which are represented by surgical, medical, radiation oncologists, as well as pathologists and radiologists. Neoadjuvant chemoradiotherapy is typically recommended in locally advanced mid to lower rectal cancers that are T3, positive nodal status, threatened circumferential radial margin on pre-operative magnetic resonance imaging (MRI).
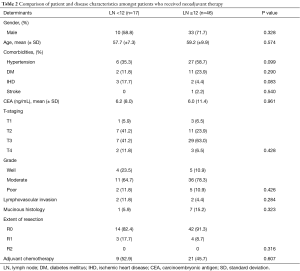
Further analysis was then conducted on patients who had received neoadjuvant chemoradiotherapy. Patients who had less than 12 lymph nodes harvested were compared to those who had 12 or more lymph nodes harvested. Patient characteristics such as gender, ethnicity, age, and the presence of comorbidities were compared between these two groups. Disease characteristics including mean carcinoembryonic antigen (CEA) values, T-staging, tumour grade, presence of lymphovascular invasion, mucinous histology, extent of resection, as well as whether the patient received adjuvant chemotherapy were also compared. Univariable comparisons of categorical data between groups were performed by Chi-square test, while continuous data were analysed by Student’s t-test. Statistical analysis was performed using Stata software, version 14 (StataCorp, College Station, TX, USA).
All patients were followed up at the outpatient surgical clinic and in concordance to guidelines by American Gastroenterology Association (9). All patients received regular history and physical examination, as well as endoscopic and radiological investigations as needed to identify recurrences. Oncologic endpoints of recurrence and survival were then analysed. Overall survival and recurrence outcomes were analysed by the Kaplan-Meier method, whilst recurrence and disease-free survival (DFS) were analysed by multivariate Cox regression. The study was approved by the National Healthcare Group Domain Specific Review Board (NHG DSRB). NHG DSRB reference number 2015/00842 and informed consent was taken from all patients.
Results
Between January 2008 and December 2014, there were 217 patients who underwent surgical resection for rectal cancer. Out of this, 63 (29.0%) had received neoadjuvant therapy prior to surgical resection. Seventeen (27.0%) patients from the neoadjuvant therapy group had less than twelve lymph nodes in the final resection specimen compared to 14 (9.1%) patients (P=0.001) who did not receive neoadjuvant chemotherapy. Mean lymph node yield was also significantly less amongst patients who had received neoadjuvant therapy compared with those who did not (14.5±6.1 vs. 17.3±5.6, P=0.002) (Table 1).

Full table
Further analysis was conducted on the group of patients who received neoadjuvant therapy. Patient and disease characteristics were compared between lymph node yields of less than 12 lymph nodes and lymph node yields of 12 and more lymph nodes (Table 2). Gender (male sex: 58.8% vs. 71.7%, P=0.328), ethnicity, mean age (57.7±7.3 vs. 59.2±9.9, P=0.574), and presence of comorbidity were not statistically significantly different between both groups. Disease characteristics such as mean CEA levels (6.2 vs. 6.0, P=0.961), T-staging, grade, presence of lymphovascular invasion (11.8% vs. 4.4%, P=0.284), mucinous histology (5.9% vs. 15.2%, P=0.323) extent of resection, as well as administration of adjuvant chemotherapy (52.9% vs. 45.7%, P=0.607) were also found to have no statistically significant difference between the 2 groups.
Full table
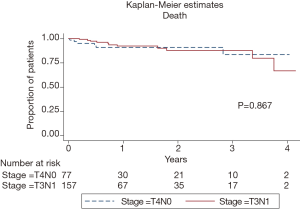
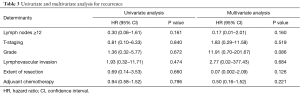
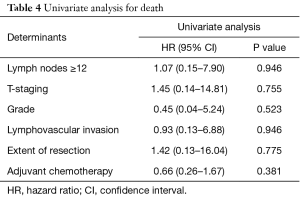
These patients were followed up for a mean of 23.4 (interquartile range, 9–40.5) months. Univariate and multivariate analysis of lymph node yield together with disease variables which included T-staging, histological grade, lymphovascular invasion, extent of resection and administration of adjuvant chemotherapy were conducted for both recurrence as well as overall survival. On multivariate analysis, lymph node yield of more than or equal to 12 was not associated with a statistically significant difference in time to recurrence [hazard ratio (HR) 0.17; 95% confidence interval (CI), 0.01–2.01, P=0.160] (Table 3). Multivariate analysis for overall survival could not be performed due to small numbers of patients who were deceased in our population. However, on univariate analysis, we noted that there was also no statistically significant difference in time to death with a lymph node yield of more than or equal to 12 lymph nodes (HR 1.07; 95% CI, 0.15–7.90, P=0.946) (Table 4). Kaplan-Meier curves for recurrence and death comparing lymph node yields of less than twelve and more than or equal to twelve were also performed and both were found to not be statistically significantly different (recurrence: P=0.203; death: P=0.867) (Figures 1,2).
Full table
Full table
Discussion
Our results confirm that whilst there is a reduced lymph node yield amongst rectal cancer patients who have received neoadjuvant chemotherapy, but yet this reduced lymph node yield did not lead to any difference in the subsequent oncological outcomes. To note, there were no intrinsic patient or disease characteristics which may have led to the differences in the lymph node yields. Multivariate analysis of time to disease recurrence further showed that even when controlling for the various histopathological determinants, there was no statistically significant difference in the time to recurrence.
The results from our findings add further weight to the literature that achieving 12 lymph nodes for accurate staging following neoadjuvant chemoradiotherapy for locally advanced rectal cancer may not be required. In fact, the presence of fewer lymph nodes in the final resection specimen following neoadjuvant therapy could imply improved response leading to better tumour regression, and possibly better long-term oncologic results (10). Studies have demonstrated a lower lymph node yield and PCR in the rectum following neoadjuvant therapy (11,12). It was postulated that improved tumour response towards neoadjuvant therapy leads to both reduction in the burden of disease in the lymph nodes as well as at the primary tumour site.
Confusion in the requirement to retrieve at least 12 lymph nodes exists primarily because there is no concession to this requirement in rectal cancer patients who have received neoadjuvant therapy. As such, many authors have proposed novel methods to increase lymph node yield during the surgical phase (13), or post-operatively during pathological analysis (14,15). Our findings therefore suggest that such a requirement is not necessary in the accurate staging and prognostication of rectal cancer. This would reduce the burden and need to artificially maximise the retrieval of lymph nodes from the surgical specimen using adjunctive techniques. Besides, in an oncological resection for rectal cancer, the higher number of lymph nodes that are harvested could have originated in proximity to the inferior mesenteric artery from a wider area of resection rather than from the mesorectum.
Limitations to our study included the small sample size and the retrospective study design which, as discussed, precluded multivariate analysis of overall survival in our patient groups. Although this raises the risk of committing a type two error in the final analysis of our results, our findings do concur with a large body of literature which has suggested similar results. We also noted that owing to the retrospective nature of our study that differences in surgical as well as histopathological techniques may have contributed to differences in lymph node harvesting.
Conclusions
Although lymph node yield is reduced in rectal cancer patients who have received neoadjuvant therapy, our findings suggest that this exhibits no impact on oncologic outcomes. The requirement to harvest at least 12 lymph nodes for accurate staging in rectal cancers following neoadjuvant chemoradiotherapy need to be explored in future studies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the National Healthcare Group Domain Specific Review Board (NHG DSRB). NHG DSRB reference number 2015/00842 and informed consent was taken from all patients.
References
- Xu Z, Berho ME, Becerra AZ, et al. Lymph node yield is an independent predictor of survival in rectal cancer regardless of receipt of neoadjuvant therapy. J Clin Pathol 2017;70:584-92. [Crossref] [PubMed]
- Amajoyi R, Lee Y, Recio PJ, et al. Neoadjuvant therapy for rectal cancer decreases the number of lymph nodes harvested in operative specimens. Am J Surg 2013;205:289-92. [Crossref] [PubMed]
- Damin DC, Rosito MA, Contu PC, et al. Lymph node retrieval after preoperative chemoradiotherapy for rectal cancer. J Gastrointest Surg 2012;16:1573-80. [Crossref] [PubMed]
- Mechera R, Schuster T, Rosenberg R, et al. Lymph node yield after rectal resection in patients treated with neoadjuvant radiation for rectal cancer: A systematic review and meta-analysis. Eur J Cancer 2017;72:84-94. [Crossref] [PubMed]
- Lykke J, Jess P, Roikjaer O, et al. A minimum yield of twelve lymph nodes in rectal cancer remains valid in the era of neo-adjuvant treatment: results from a national cohort study. Int J Colorectal Dis 2015;30:347-51. [Crossref] [PubMed]
- Gurawalia J, Dev K, Nayak SP, et al. Less than 12 lymph nodes in the surgical specimen after neoadjuvant chemo-radiotherapy: an indicator of tumor regression in locally advanced rectal cancer? J Gastrointest Oncol 2016;7:946-57. [Crossref] [PubMed]
- Kim HJ, Jo JS, Lee SY, et al. Low Lymph Node Retrieval After Preoperative Chemoradiation for Rectal Cancer is Associated with Improved Prognosis in Patients with a Good Tumor Response. Ann Surg Oncol 2015;22:2075-81. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- American Gastroenterology Association. AGA institute guidelines for colonoscopy surveillance after cancer resection: clinical decision tool. Gastroenterology 2014;146:1413-4. [Crossref] [PubMed]
- Bustamante-Lopez L, Nahas CS, Nahas SC, et al. Understanding the factors associated with reduction in the number of lymph nodes in rectal cancer patients treated by neoadjuvant treatment. Int J Colorectal Dis 2017;32:925-7. [Crossref] [PubMed]
- Runau F, Collins A, Fenech GA, et al. A single institution's long-term follow-up of patients with pathological complete response in locally advanced rectal adenocarcinoma following neoadjuvant chemoradiotherapy. Int J Colorectal Dis 2017;32:341-8. [Crossref] [PubMed]
- Persiani R, Biondi A, Gambacorta MA, et al. Prognostic implications of the lymph node count after neoadjuvant treatment for rectal cancer. Br J Surg 2014;101:133-42. [Crossref] [PubMed]
- Wang Y, Deng H, Chen H, et al. Preoperative Submucosal Injection of Carbon Nanoparticles Improves Lymph Node Staging Accuracy in Rectal Cancer after Neoadjuvant Chemoradiotherapy. J Am Coll Surg 2015;221:923-30. [Crossref] [PubMed]
- Gehoff A, Basten O, Sprenger T, et al. Optimal lymph node harvest in rectal cancer (UICC stages II and III) after preoperative 5-FU-based radiochemotherapy. Acetone compression is a new and highly efficient method. Am J Surg Pathol 2012;36:202-13. [Crossref] [PubMed]
- Sinan H, Demirbas S, Ersoz N, et al. Who is responsible for inadequate lymph node retrieval after colorectal surgery: surgeon or pathologist? Acta Chir Belg 2012;112:200-8. [Crossref] [PubMed]