Curative treatment for metastatic colorectal cancer in the young population: is it worth it?
Introduction
Colorectal cancer (CRC) is the commonest cancer in Singapore and accounts for significant morbidity and mortality (1). A significant proportion of patients with CRC present prior to 50 years old and these individuals often represent the most economically productive population in any society (2-5). Of those who developed CRC when they are young, a significant proportion of them often also present with advanced disease (2-5) with more than 20% of them having metastatic disease on diagnosis (6,7).
There has been growing interest in the management of metastatic CRC in recent years, and the boundaries of curative surgery in this group of patients continue to be redefined. Young patients with metastatic CRC are often treated aggressively to provide them with the best chance of cure. Although these younger patients are often medically healthy and have greater functional reserves, surgery and its accompanying systemic or local treatment are not without its implications. These risks should only be justified if there are truly significant improvements in survival and quality of life, with an acceptable rate of recurrence.
The aim of this study is to review the management and outcomes of young patients with CRC who presented with metastatic disease. Whilst pursuing curative treatment appears promising and instinctive, physicians ought to give patients and families realistic expectations of treatment outcomes, and also consider the psychosocial and financial burdens that patients and family face when counselling them on the treatment options.
Methods
A retrospective review of all patients, who were under the age of 50 years, diagnosed with metastatic CRC in a single institution from January 2007 to December 2015 was conducted. These patients were identified from a pre-existing prospectively maintained colorectal cancer database. Confirmation of the malignancy was achieved histologically while staging was performed with computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET) or a combination of the above.
The management of each patient was discussed at a multi-disciplinary tumor board, consisting of a team of medical oncologist, radiation oncologists, surgeons and radiologists. All patients who underwent curative surgery subsequently had adjuvant chemotherapy.
Data collected included demographics, clinical presentations, disease characteristics, treatment received and outcomes. Results were analyzed with SPSS version 21.0 and P<0.05 was considered statistically significant. Univariate analysis was performed using Mann-Whitney U test for continuous variables and Pearson’s Chi square or Fisher’s Exact test for categorical variables. Cumulative overall survival rate was calculated by the Kaplan-Meier method and compared by the log-rank test. The study was approved by the National Healthcare Group’s Domain Specific Research Board, reference number 2015/00842.
Results
From January 2007 to December 2015, a total of 1,367 patients were diagnosed with CRC in our institution, of which 154 patients (11.3%) were under the age of 50, of which 33 (21.4%) had stage IV disease.
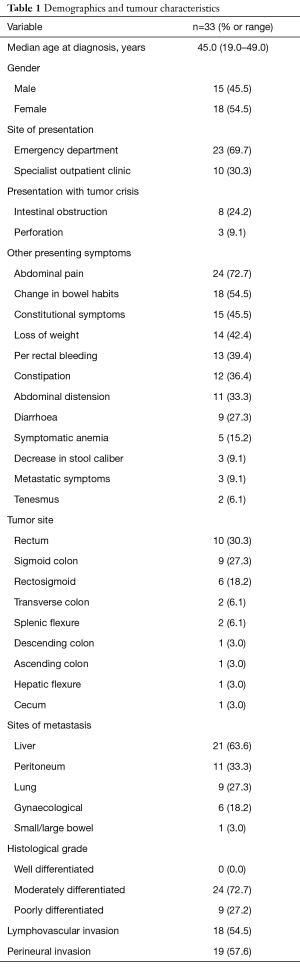
The median age at diagnosis for the study group was 45 years (range, 19–49 years). Majority of the patients (n=23, 69.7%) first presented to the emergency department (ED). Amongst them, 11 patients (47.8%) presented with intestinal obstruction or perforation from the cancer. Table 1 provides further details on the demographics and clinical presentations of our study population.
Full table
The majority of the patients (n=28, 84.8%) had left sided cancers. Nine patients (27.3%) had more than 1 site of metastasis. Information on tumor location and histopathologic variables are found in Table 1.
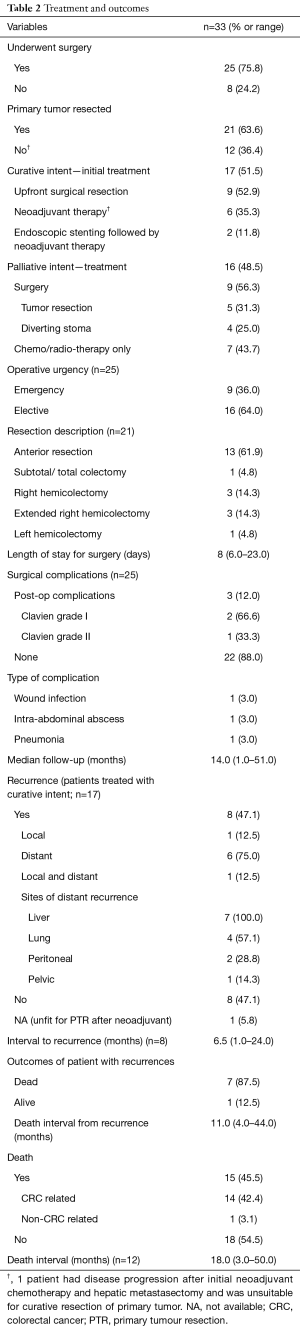
Twenty-five (75.8%) patients underwent surgery, of which 17 (68%) were with curative intent. Of the 17 patients (51.5%) who were treated with curative intent, 9 (52.9%) patients underwent upfront surgical resection followed by adjuvant treatment, 6 (35.3%) patients had a trial of neoadjuvant therapy prior to surgical resection and 2 (11.8%) patients had successful endoscopic stents followed by a trial of neoadjuvant treatment. Among the 16 (48.5%) patients treated with palliative intent, 9 (56.3%) patients underwent surgery to either have their tumour resected or creation of a diverting stoma. Table 2 illustrates the treatment details of our study population.
Full table
Among the 25 patients who underwent surgery, the median length of hospitalisation was 8 days (range, 6–23 days). None of them experienced a surgical morbidity of Clavien III and above. Patients were followed up for a median duration of 14.0 months (range, 1.0–51.0 months).
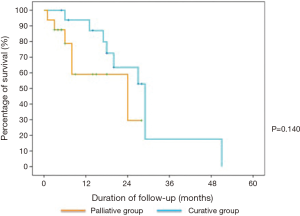
Among the 17 patients treated with curative intent, 8 (47.1%) had disease recurrence, majority were distant recurrences, with a median time to recurrence of 6.5 months (range, 1.0–24.0 months). There were 14 (42.4%) CRC-related mortalities in our study population, and the median time to death from diagnosis was 18.0 months (range, 3.0–50.0 months). There was no significant difference in median survival between the patients who underwent curative treatment and those who had palliation (29 vs. 24 months, P=0.140) as illustrated in Table 3 as well as in the Kaplan-Meier survival curve in Figure 1. The outcomes of our study population are shown in Table 2.

Full table
Discussion
The overall incidence of CRC in the general population has declined in recent years, and this is largely attributed to the increase in proportion of population undergoing screening and the removal of pre-malignant lesions (8,9). However, the incidence of CRC in the population younger than 50 years old appears to be increasing (2-5,8). A possible explanation is the rise in incidence of CRC risk factors among the young, such as metabolic syndromes like diabetes mellitus and obesity (9,10). Although there is increasing data on the clinical presentations, disease characteristics and outcomes of young CRC patients (2-5,11-16), there remains a scarcity of information on the subgroup of patients with metastatic disease.
Once believed to be a terminal disease with a dismal overall survival (17), metastatic CRC is now potentially curable in a select group of patients, achieving 5-year survival rates of up to 45% (18). Various studies have reported survival and quality of life benefits in patients with metastatic CRC who undergo curative surgery (18-22). Younger patients, whom are associated with more advanced and aggressive diseases do possess better oncological outcomes than their older counterparts (23). This is observed even amongst patients with metastatic disease (23-27). This may thus support the push for aggressive potentially curative treatment in the younger age group.
There are several important observations from our study. Firstly, patients who undergo treatment with a palliative intent do have a reasonable median survival of 2 years. The advent of newer chemotherapeutic and immunological agents would have accounted for this improved survival over the years. In these individuals with extensive and unresectable systemic disease, the intention of primary resection of the tumour could be reserved for alleviation of symptoms and prevention or life-saving treatment of tumor crisis such as obstruction, perforation or bleeding. It was perhaps fortunate that none of our patients who underwent surgery had any significant morbidity. This could be because patients in this age group are often healthy and have greater functional reserves
In addition, the median survival of patients who underwent treatment with curative intent, including surgery, was nearly 30 months in our study. Perhaps one could downplay the significance of a 5-month improvement in overall survival that is conferred by curative treatment, but these 5 months could be extremely important to these individuals, especially in those who have yet come to acceptance and needs the time to make plans for those they are leaving behind upon demise. That said, whether the associated morbidities and impediments to their quality of life from the repeated surgeries (to the primary and the systematic disease) and perhaps carrying a stoma were worthy of these 5 months merits further evaluation.
Over treatment is a genuine concern in the management of these young mCRC patients. Oncologists and surgeons tend to be far more aggressive in the management of these younger adults, and often attempt to deplete the arsenal of treatment options before giving in to palliative care. This is likely because these adults are in the most economically productive phase of their lives and have great potential in the years ahead, and the attending surgeons and oncologists often become emotionally attached to these patients. Costly treatments, including the use of immunological therapy may only marginally improve survival but places huge financial and psychosocial burdens on the patient and family. It may be worthwhile to explore palliative options early instead of attempting to exhaust all available treatment options with no intent of closure or accepting the inevitable demise of these patients.
Although attempting and pushing for curative treatment is often the instinctive choice in the management of young patients with metastatic CRC, and though it may truly render half of these patients disease free, it is imperative to remember that the barrage of treatments will fail in the other half of these patients and at the same time place a significant emotional and financial burden on the patient and family. It is therefore the responsibility of the attending physician to spend the time and effort to counsel these patients and their families extensively to ensure that goals of therapy are clearly defined and achievable.
The various limitations of our study include it being a retrospective study with its own inherent biases. The small sample size and short duration of follow-up may also limit the validity of our study. However, young patients who present with metastatic CRC are uncommon and it is difficult to conduct large-scale studies for this group of patients. Although this is a highly selected group of patients, they represent an important population that should not be neglected. It is thus important to conduct future prospective studies with the aim of better selecting patients who will benefit the most from treatment with curative intent.
Conclusions
Young CRC patients with stage IV disease typically survive for 2 years upon diagnosis. Those who were treated with curative intent have a slightly longer and not statistically significant median survival than those treated with palliative intent. The role of aggressive treatment in these young patients with metastatic patients merits further evaluation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the National Healthcare Group’s Domain Specific Research Board, reference number 2015/00842.
References
- Singapore Cancer Registry Annual Registry Report Trends in Cancer Incidence in Singapore 2010–2014. Available online: https://www.nrdo.gov.sg/docs/librariesprovider3/default-document-library/cancer-trends-report-2010---2014_web.pdf?sfvrsn=0
- O’Connell JB, Maggard MA, Liu JH, et al. Rates of colon and rectal cancers are increasing in young adults. Am Surg 2003;69:866-72. [PubMed]
- Amri R, Bordeianou LG, Berger DL. The conundrum of the young colon cancer patient. Surgery 2015;158:1696-703. [Crossref] [PubMed]
- You YN, Xing Y, Feig BW, et al. Young-onset colorectal cancer: is it time to pay attention? Arch Intern Med 2012;172:287-9. [Crossref] [PubMed]
- Ahnen DJ, Wade SW, Jones WF, et al. The increasing incidence of young onset colorectal cancer: a call to action. Mayo Clin Proc 2014;89:216-24. [Crossref] [PubMed]
- Myers EA, Feingold DL, Forde KA, et al. Colorectal cancer in patients under 50 years of age: A retrospective analysis of two institutions’ experience. World J Gastroenterol 2013;19:5651-7. [Crossref] [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [Crossref] [PubMed]
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544-73. [Crossref] [PubMed]
- Sarfaty M, Myers RE. The effect of HEDIS measurement of colorectal cancer screening on insurance plans in Pennsylvania. Am J Manag Care 2008;14:277-82. [PubMed]
- Yuhara H, Steinmaus C, Cohen SE, et al. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol 2011;106:1911-21. [Crossref] [PubMed]
- McKay A, Donaleshen J, Helewa RM, et al. Does young age influence the prognosis of colorectal cancer: a population-based analysis. World J Surg Oncol 2014;12:370. [Crossref] [PubMed]
- Liang J, Kalady MF, Church J. Young age of onset colorectal cancers. Int J Colorectal Dis 2015;30:1653-7. [Crossref] [PubMed]
- Zbuk K, Sidebotham EI, Bleyer A, et al. Colorectal cancer in young adults. Semin Oncol 2009;36:439-50. [Crossref] [PubMed]
- de Sousa JB, Souza CS, Fernandes MB, et al. Do young patients have different clinical presentation of colorectal cancer causing delay in diagnosis? Int J Colorectal Dis 2014;29:519-27. [Crossref] [PubMed]
- Ho YH, Siu SK, Buttner P, et al. The effect of obstruction and perforation on colorectal cancer disease-free survival. World J Surg 2010;34:1091-101. [Crossref] [PubMed]
- Law JH, Koh FH, Tan KK. Young colorectal cancer patients often present too late. Int J Colorectal Dis 2017;32:1165-9. [Crossref] [PubMed]
- Bird NC, Mangnall D, Majeed AW. Biology of colorectal liver metastases: A review. J Surg Oncol 2006;94:68-80. [Crossref] [PubMed]
- Kanas GP, Taylor A, Primrose JN, et al. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol 2012;4:283-301. [PubMed]
- Yancik R. Population aging and cancer: a cross-national concern. Cancer J 2005;11:437-41. [Crossref] [PubMed]
- UK Colorectal Cancer Screening Pilot Group. Results of the first round of a demonstration pilot of screening for colorectal cancer in the United Kingdom. BMJ 2004;329:133. [Crossref] [PubMed]
- Chan KK, Dassanayake B, Deen R, et al. Young patients with colorectal cancer have poor survival in the first twenty months after operation and predictable survival in the medium and long-term: analysis of survival and prognostic markers. World J Surg Oncol 2010;8:82. [Crossref] [PubMed]
- Tan WJ, Chew MH, Tan IB, et al. Palliative surgical intervention in metastatic colorectal carcinoma: a prospective analysis of quality of life. Colorectal Dis 2016;18:357-63. [Crossref] [PubMed]
- Wang R, Wang MJ. Clinicopathological Features and Survival Outcomes of Colorectal Cancer in Young Versus Elderly: A Population-Based Cohort Study of SEER 9 Registries Data (1988–2011). Medicine (Baltimore) 2015;94:e1402. [Crossref] [PubMed]
- O'Connell JB, Maggard MA, Liu JH, et al. Do young colon cancer patients have worse outcomes? World J Surg 2004;28:558-62. [Crossref] [PubMed]
- Enblad G, Enblad P, Adami HO, et al. Relationship between age and survival in cancer of the colon and rectum with special reference to patients less than 40 years of age. Br J Surg 1990;77:611-6. [Crossref] [PubMed]
- Turkiewicz D, Miller B, Schache D, et al. Young patients with colorectal cancer: how do they fare? ANZ J Surg 2001;71:707-10. [Crossref] [PubMed]
- Hawk NN, Long TE, Imam MH, et al. Clinicopathologic features and outcome of young adults with stage IV colorectal cancer. Am J Clin Oncol 2015;38:543-9. [Crossref] [PubMed]