Rapidly progressive subcutaneous metastases from gallbladder cancer: insight into a rare presentation in gastrointestinal malignancies
Introduction
Gallbladder cancer (GBCA) is the sixth most common cancer of the gastrointestinal (GI) tract and the most common cancer of the biliary tree (1). This malignancy is relatively rare with an annual age-adjusted incidence of 1.2 cases per 100,000 people (1,2). Furthermore, GBCA carries a poor prognosis with 5-year overall survival rates of 0.5-12% and median survival of 2-19 months, depending on the stage of disease (2-4). Like other GI cancers, the risk of GBCA increases with age (5). However, unlike other GI malignancies, the incidence of GBCA is approximately 3-fold higher in women than in men (2,5,6). Moreover, the incidence of GBCA also varies based on geographic region and ethnicity suggesting that both environmental and genetic factors influence the etiology of this disease (6-8). In the Western world, cholelithiasis and chronic cholecystitis are the most common predisposing factors associated with GBCA (9). In fact, 70-95% of all patients with GBCA have gallstones and a history of cholelithiasis leads to a 34-fold higher risk of developing GBCA (1,3,10-12). While the overall incidence of GBCA is <0.2% in patients with cholelithiasis, GBCA should be entertained in the differential diagnosis of patients presenting with calcified and thickened gallbladder with cholelithiasis. As a result, patients with GBCA may present with signs and symptoms of cholecystitis or vague right upper quadrant pain. In turn, most patients are diagnosed with more advanced stages of GBCA with spread to both local and distant sites (1-4,13). The most common modes of spread are: (I) direct extension into the liver and porta hepatitis; (II) spread into local and regional lymph nodes; (III) peritoneal seeding which can lead to carcinomatosis, ascites, and biopsy/surgical site occurrences; and (IV) hematogenous spread to distant sites including non-contiguous sites in the liver (2-4,14-16). More rarely, hematogenous spread can result in distant metastases to the lungs and brain (15,17). Finally, GBCA can also spread along nerves and within the biliary tree (15). Metastases to distant subcutaneous tissue are exceedingly rare with only six reported cases of GBCA with metastases to subcutaneous tissue (18-23). Interestingly, 5 of 6 (83.3%) cases involved breast metastases while 2 of 6 (33.3%) cases involved chest/axilla metastases. We now present the first reported case of multiple, rapidly progressive subcutaneous GBCA metastases distant from laparoscopic incision sites and the chest (i.e., buttock) and review the literature on this rare pattern of metastases in patients with GBCA and other solid tumors. This case report will highlight some rare, but unique pitfalls in the diagnosis of this GI malignancy.
Case
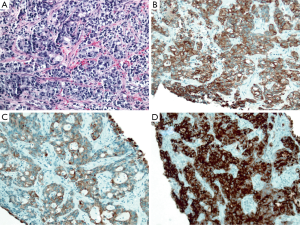
A previously healthy 58-year old Caucasian female presented with acute right-sided abdominal pain radiating into her right lower quadrant. An ultrasound of the right upper quadrant revealed gallstones in a distended gallbladder with wall thickening to 7.4 mm. A subsequent computed tomography (CT) scan of the abdomen and pelvis demonstrated a 4.3 cm calcified gallstone in a distended gallbladder with edema within the wall. Cholescintigraphy with a hepatobiliary iminodiacetic acid (HIDA) scan showed non-visualization of the gallbladder. Therefore, the patient was taken to the operating room for diagnostic laparoscopy and planned cholecystectomy. At the time of surgery, a firm, indurated mass was identified in the right upper quadrant of the abdomen. This involved the gallbladder and was adherent to adjacent omentum. Biopsies of the gallbladder mass were obtained. Pathological review revealed moderately-to-poorly differentiated adenocarcinoma (Figure 1A). Immunohistochemical stains showed tumor cells were diffusely positive for cytokeratin 7 (CK7) (Figure 1B), cytokeratin 19 (CK19) (Figure 1C), carcinoembryonic antigen (CEA) (Figure 1D), and pancytokeratin (data not shown). The immunohistochemical staining pattern favored an adenocarcinoma of pancreatobiliary origin, consistent with a GBCA. At the time of surgery, the tumor was clinically staged as a Stage IIIA (T3NxMx) tumor.
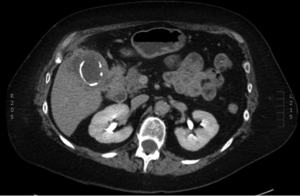
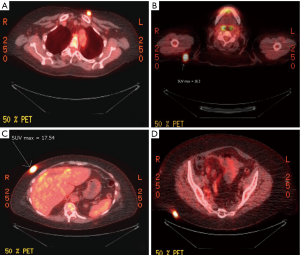
Upon recovery, the patient was referred to our center for complete staging workup and possible operative management. Initial physical examination was notable for her healing abdominal incisions and the absence of palpable abdominal masses or hepatomegaly. Subcutaneous nodules were not clinically evident. In order to fully stage her disease, a PET/CT of the chest, abdomen, and pelvis was obtained. This revealed intense focal uptake around the gallbladder consistent with the region of gallbladder wall thickening and hypoattenuation of the liver adjacent to the gallbladder (Figure 2). PET/CT also revealed multiple FDG-avid subcutaneous nodules in the right shoulder (Figure 3A), chest (Figure 3B), anterior abdominal wall at a location distinct from any laparoscopic port sites (Figure 3C), and the buttocks (Figure 3D). The maximum standardized uptake values (SUVs) for these lesions was 17.5. The PET/CT study also revealed an enlarged mesenteric lymph node (SUVmax 6.5) and mediastinal lymph node (SUVmax 19.4), as well as lesions in the sacrum (SUVmax 20.6) and left femoral head (images not shown). No metastatic disease was observed in the liver or peritoneal cavity.
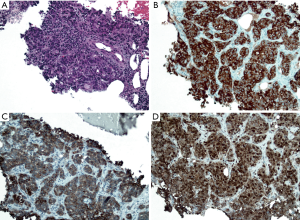
The patient returned to clinic the following week after the PET/CT was performed. At this time she described the appearance of the right shoulder, chest wall and buttocks masses over the previous weeks. Upon physical examination, these nodules were now quite prominent. In order to determine if these nodules represented a synchronous malignancy such as melanoma, lymphoma, or squamous cell carcinoma, a core needle biopsy was performed on the chest wall nodule. Pathological review revealed an adenocarcinoma (Figure 4A). Similar to the GBCA, immunohistochemical stains showed tumor cells that were diffusely positive for CK7 (Figure 4B), CK19 (Figure 4C), and CEA (Figure 4D). Again, the immunohistochemical staining pattern favored an adenocarcinoma of pancreatobiliary origin. Therefore, this was consistent with a metastatic GBCA. Given her multifocal metastatic disease, the patient was started on systemic chemotherapy with gemcitabine and cisplatin. Interval imaging performed 3 months after initiating chemotherapy showed stable disease. At the time of writing, the patient continues to receive treatment and is tolerating therapy well.
Discussion
The most common metastatic sites for GBCA are the local lymph nodes, liver, or surrounding peritoneal structures (3,15). Occasionally, extra-peritoneal metastases may occur, but these are primarily in the lungs and the pleura (24). Our patient presented with what appeared to be locally advanced disease without metastases. During the course of her staging workup, her disease rapidly progressed and PET/CT revealed several FDG-avid subcutaneous and bone metastases. When the patient returned to our clinic, the subcutaneous nodules were palpable in the right shoulder, the chest wall overlying the sternum, the left subclavicular region, the right anterior abdominal wall distant from the laparoscopic incision site, and the buttocks. Due to their rapid enlargement, there was concern that these nodules were the result of a second primary tumor, like melanoma. However, a biopsy of one of the nodules was consistent with metastatic GBCA. The current case demonstrates that GBCA has the potential to metastasize to distant subcutaneous sites. Thus, we confirm GBCA’s position on the short list of solid visceral malignancies with this potential. As such, clinicians must be aware of this rare, but unique, presentation when performing a review of systems and a thorough physical examination in patients with GBCA.
GBCA is unique in that it has many potential modes of spread. Fahim and colleagues noted that in addition to direct extension into adjacent structures, GBCA can also spread via the lymphatics, along the nerves, intraductally, or hematogenously (15). Hematogenous spread is the most likely mechanism for metastasis to distant, extra-lymphatic sites (25,26). Although the precise mechanism for subcutaneous metastases from GBCA is unknown, the appearance of these metastases in our patient can be conceptually explained by hematogenous spread. However, the pattern of hematogenous spread from biliary tree cancers is most often to the liver followed by spread to more distant sites like the lungs (26-28). To our knowledge, there are only six other similar case presentations in the literature (18-23). Including our case, 6 of 7 (85.7%) are in women and 71.4% are to breast tissue (18-22). Biologically this is a curious finding and raises the question of a hormonal chemotactic factor inducing this. Further studies are needed to confirm this hypothesis. Our patient is different in that she had no breast lesions but rather metastases to other quite remote soft tissue sites including the buttocks.
The occurrence of cutaneous and/or subcutaneous metastases arising from solid tumor malignancies is rare. Based upon autopsy studies, they are present in approximately 0-5% of cases, although some series report an incidence as high as 9-10% (9,29). This discrepancy may be explained, in part, by the fact that few studies describing skin metastases arising from visceral organs distinguish subcutaneous and cutaneous metastases. There is much less data available on the incidence of subcutaneous metastases specifically. However, a literature search for “subcutaneous metastases” on PubMed yields numerous case reports documenting subcutaneous metastases at different sites from various solid malignancies. Subcutaneous metastases have previously been reported in patients with breast cancer, genitourinary cancers, colon cancers, gastric cancers, hepatocellular carcinomas, gynecologic cancers, esophageal and thyroid cancers, and lung cancers (30-39). In light of the present case and earlier reports, GBCA must now be firmly added to the short list of solid tumor cancers that can metastasize to distant sites in the soft tissue.
GBCA is a rare but lethal cancer. Although advances have been made in the diagnosis and treatment of GBCA, this case highlights not only the aggressive nature of this cancer, but also a unique pattern of metastatic disease. Over the course of several weeks, our patient developed rapidly enlarging subcutaneous metastases and spread to several distant sites. Unlike other reported cases of subcutaneous metastases from a primary GBCA, these subcutaneous metastases did not occur at laparoscopic port sites or within the breast tissue. In light of this unique presentation, GBCA should now be included in the differential diagnosis for subcutaenous nodules in patients with this GI cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Russo MW, Wei JT, Thiny MT, et al. Digestive and liver diseases statistics, 2004. Gastroenterology 2004;126:1448-53. [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010. Available online: http://seer.cancer.gov/csr/1975_2010/
- Misra S, Chaturvedi A, Misra NC, et al. Carcinoma of the gallbladder. Lancet Oncol 2003;4:167-76. [PubMed]
- Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer 2004;4:695-706. [PubMed]
- Lazcano-Ponce EC, Miquel JF, Muñoz N, et al. Epidemiology and molecular pathology of gallbladder cancer. CA Cancer J Clin 2001;51:349-64. [PubMed]
- Castro FA, Koshiol J, Hsing AW, et al. Biliary tract cancer incidence in the United States-Demographic and temporal variations by anatomic site. Int J Cancer 2013;133:1664-71. [PubMed]
- Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer 2006;118:1591-602. [PubMed]
- Zatonski WA, Lowenfels AB, Boyle P, et al. Epidemiologic aspects of gallbladder cancer: a case-control study of the SEARCH Program of the International Agency for Research on Cancer. J Natl Cancer Inst 1997;89:1132-8. [PubMed]
- Ciombor KK, Goff LW. Current therapy and future directions in biliary tract malignancies. Curr Treat Options Oncol 2013;14:337-49. [PubMed]
- Hart J, Modan B, Shani M. Cholelithiasis in the aetiology of gallbladder neoplasms. Lancet 1971;1:1151-3. [PubMed]
- Chen YK, Yeh JH, Lin CL, et al. Cancer risk in patients with cholelithiasis and after cholecystectomy: a nationwide cohort study. J Gastroenterol 2014;49:923-31. [PubMed]
- Bosman FT, Carneiro F, Hruban RH, et al. eds. WHO Classification Of Tumours Of The Digestive System. 4th Edition. Lyon: IARC Press, 2010.
- D’Hondt M, Lapointe R, Benamira Z, et al. Carcinoma of the gallbladder: patterns of presentation, prognostic factors and survival rate. An 11-year single centre experience. Eur J Surg Oncol 2013;39:548-53. [PubMed]
- Chikamoto A, Tsuji T, Nakahara O, et al. Cancer cells spread through lymph vessels in the submucosal layer of the common bile duct in gallbladder carcinoma. J Hepatobiliary Pancreat Surg 2009;16:557-61. [PubMed]
- Fahim RB, McDonald JR, Richards JC, et al. Carcinoma of the gallbladder: a study of its modes of spread. Ann Surg 1962;156:114-24. [PubMed]
- Baer HU, Metzger A, Glättli A, et al. Subcutaneous periumbilical metastasis of a gallbladder carcinoma after laparoscopic cholecystectomy. Surg Laparosc Endosc 1995;5:59-63. [PubMed]
- Kondo S, Nimura Y, Kamiya J, et al. Mode of tumor spread and surgical strategy in gallbladder carcinoma. Langenbecks Arch Surg 2002;387:222-8. [PubMed]
- Garg PK, Khurana N, Hadke NS. Subcutaneous and breast metastasis from asymptomatic gallbladder carcinoma. Hepatobiliary Pancreat Dis Int 2009;8:209-11. [PubMed]
- Khangembam BC, Sharma P, Naswa N, et al. Solitary breast metastasis from recurrent gallbladder carcinoma simulating a second primary on 18F-FDG PET/CT. Clin Nucl Med 2013;38:e433-4. [PubMed]
- Jeyaraj P, Sio TT, Iott MJ. An unusual case of isolated, serial metastases of gallbladder carcinoma involving the chest wall, axilla, breast and lung parenchyma. Rare Tumors 2013;5:e7. [PubMed]
- Singh S, Gupta P, Khanna R, et al. Simultaneous breast and ovarian metastasis from gallbladder carcinoma. Hepatobiliary Pancreat Dis Int 2010;9:553-4. [PubMed]
- Kallianpur AA, Shukla NK, Deo SV, et al. A rare case of gallbladder carcinoma metastases to the breast treated with curative intent. Trop Gastroenterol 2012;33:155-8. [PubMed]
- Wollina U, Graefe T, Konrad H, et al. Cutaneous metastases of internal cancer. Acta Dermatoven APA 2004;13:79-84.
- Miller G, Jarnagin WR. Gallbladder carcinoma. Eur J Surg Oncol 2008;34:306-12. [PubMed]
- Chambers AF, Naumov GN, Varghese HJ, et al. Critical steps in hematogenous metastasis: an overview. Surg Oncol Clin N Am 2001;10:243-55. [PubMed]
- Weiss L. Comments on hematogenous metastatic patterns in humans as revealed by autopsy. Clin Exp Metastasis 1992;10:191-9. [PubMed]
- Louha M, Nicolet J, Zylberberg H, et al. Liver resection and needle liver biopsy cause hematogenous dissemination of liver cells. Hepatology 1999;29:879-82. [PubMed]
- Tímár J, Csuka O, Orosz Z, et al. Molecular pathology of tumor metastasis. II. Molecular staging and differential diagnosis. Pathol Oncol Res 2002;8:204-19. [PubMed]
- Bansal R, Patel T, Sarin J, et al. Cutaneous and subcutaneous metastases from internal malignancies: an analysis of cases diagnosed by fine needle aspiration. Diagn Cytopathol 2011;39:882-7. [PubMed]
- Cormio G, Capotorto M, Di Vagno G, et al. Skin metastases in ovarian carcinoma: a report of nine cases and a review of the literature. Gynecol Oncol 2003;90:682-5. [PubMed]
- Dirican A, Küçükzeybek Y, Somal I, et al. Cutaneous and subcutaneous metastases from bladder carcinoma. Contemp Oncol (Pozn) 2012;16:451-2. [PubMed]
- Dreizen S, Dhingra HM, Chiuten DF, et al. Cutaneous and subcutaneous metastases of lung cancer. Clinical characteristics. Postgrad Med 1986;80:111-6. [PubMed]
- Gogalniceanu P, Jarral OA, Purkayastha S, et al. Chest wall metastasis from oesophageal adenocarcinoma: a rare presentation. Updates Surg 2011;63:223-6. [PubMed]
- Harisankar CN. Widespread subcutaneous metastases in a patient with breast cancer: Evaluation with fluoro deoxy-glucose positron emission tomography-computed tomography. Indian J Nucl Med 2013;28:190-1. [PubMed]
- Karyagar S, Karyagar SS, Kece C, et al. Subcutaneous metastases of colorectal cancer detected with PET/CT. Clin Nucl Med 2010;35:267-8. [PubMed]
- Katske FA, Waisman J, Lupu AN. Cutaneous and subcutaneous metastases from carcinoma of prostate. Urology 1982;19:373-6. [PubMed]
- Lo Russo G, Accarpio F, Spinelli GP, et al. Subcutaneous metastases from colon cancer: a case report. J Med Case Rep 2012;6:212. [PubMed]
- Norman JL, Cunningham PJ, Cleveland BR. Skin and subcutaneous metastases from gastrointestinal carcinoid tumors. Arch Surg 1971;103:767-9. [PubMed]
- Tezcan Y, Koc M. Hepatocellular carcinoma with subcutaneous metastasis of the scalp. Radiol Oncol 2011;45:292-5. [PubMed]


