Tailored treatment of patients with hepatocellular carcinoma with portal vein invasion: experience from a multidisciplinary hepatobiliary tumor program within a NCI comprehensive cancer center
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer mortality worldwide. It is currently the seventh largest cause of death in the United States (1), but projected to become third largest by 2030 (2). Patients with HCC presenting with portal vein involvement are very challenging to treat. These patients, classified as Barcelona-clinic liver cancer (BCLC) stage C, have an extremely poor prognosis with a median survival of only 6.9 months with combination chemotherapy (3). In the United States, the widely accepted treatment algorithm (4) is palliative systemic chemotherapy with sorafenib, based on prolonged overall survival and time to disease progression in phase 3 trials (5,6). Liver-directed treatments including surgical resection, transarterial chemoembolization (TACE), and transarterial radioembolization [intra-arterial brachytherapy (IAB)] microspheres have been traditionally contraindicated in these patients with advanced disease (4). Because more than 90% of patients with PVI have compromised liver function, liver-directed therapy can be associated with significant liver decompensation causing ascites, jaundice, and death (4,7,8). This risk of liver failure mandates great caution when selecting patients with PVI for liver-directed treatment and leads to a therapeutic nihilism surrounding HCC patients with PVI; however recent data from Europe and Asia demonstrate a survival benefit with these alternative treatments compared to sorafenib or supportive care when delivered to selected patients (9-13).
Luo et al. 2011 (10) found that extent of portal vein tumor, tumor size, and serum bilirubin were independent predictors of survival following TACE and Chung et al. 2011 (9) found that repeated TACE treatments and CTP A status independently predicted favorable outcomes in patients with HCC and portal vein tumor thrombus. We were interested in examining these variables in a Western patient population.
Our institution uses a multidisciplinary strategy to coordinate care for patients with HCC with a tumor board composed of hepatologists, medical oncologists, interventional radiologists, body imaging radiologists, surgical oncologists, liver transplant and hepatobiliary surgeons, palliative care specialists, social workers, a nurse coordinator, and a database manager. The input from this diverse group of specialists is particularly important for patients with a significant burden of disease and poor prognosis. Our working hypothesis was that we could identify characteristics associated with selection for liver-directed therapy in patients with advanced stage HCC with PVI, and define the safety and outcomes when liver-directed therapy was delivered to a selected group of HCC patients with PVI.
Methods
Patients
All consecutive patients between the ages of 18 and 95 who were referred to our institution for treatment of HCC, found to have portal vein invasion (PVI), and consented to participate in the Oregon Health & Science University liver tumor database between October 1, 2009 and June 30, 2015 were included. There were no exclusions for differences in gender, race, or ethnic origin. Patient, tumor, treatment, and follow-up data were obtained from review of patient records. PVI was classified using Vp0–Vp4 grades (14,15) proposed by the Liver Cancer Study Group of Japan and outlined by Katagiri et al. 2014 (14): “Vp0, no tumor thrombus in the portal vein; Vp1, presence of a tumor thrombus distal to, but not in, the second-order branches of the portal vein; Vp2, presence of a tumor thrombus in the second-order branches of the portal vein; Vp3, presence of a tumor thrombus in the first-order branches of the portal vein; and Vp4, presence of a tumor thrombus in the main trunk of the portal vein or a portal vein branch contralateral to the primarily involved lobe (or both).”
Approval of the Institutional Review Board at Oregon Health & Sciences University was obtained. Informed consent was obtained from all participants. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 1983.
Treatments
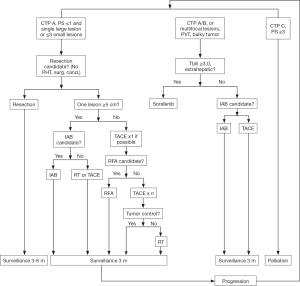
Treatment recommendations were made based on review of each case by a multidisciplinary liver tumor board at the time portal vein tumor thrombus was diagnosed. Patients who had been previously presented at the time of HCC diagnosis were discussed again when portal vein involvement was discovered. Figure 1 depicts our current HCC treatment algorithm, which has evolved since the study period to include patients with bilateral disease. In general during the study period, patients who met CTP A criteria or had larger tumors were referred for selective or lobar IAB in the absence of other contraindications. The target dose was usually 120–140 Gray for bilateral disease, or as high as 200 Gray if segmental or lobar treatment was feasible given the distribution of disease. Patients who were categorized as CTP B or CTP A with a shunt fraction prohibitive for IAB, or patients with single relatively small tumors, were referred for selective TACE. After this decision point, an initial treatment algorithm was designed to address the entire tumor burden, which may have included planned staged treatments in patients with extensive disease to decrease the risk of acute hepatic failure. Patients referred for liver-directed therapy were treated every four weeks as long as liver function was preserved until the entire extent of disease burden had been addressed. After completion of the initial treatment course, patients were followed with repeat imaging and liver function blood tests at one month, then every three months for two years, then every six months. If a patient responded well to an initial treatment modality, this modality was repeated in the event of recurrence unless new contraindications were present. Treatment decisions were updated on a regular basis, and patients were represented at multidisciplinary tumor board as new information became available including response to treatments, changes in performance status or comorbidities, or disease progression. Contraindications to liver-directed therapies are outlined in Table 1.

Full table
Because of the relatively small sample size, the patients were divided into two groups for analysis. One group (systemic) included all patients not referred for liver-directed therapy. This group consisted of patients treated with sorafenib and patients who elected to receive palliative or supportive care while the other group (liver-directed) consisted of all patients who underwent at least one liver-directed treatment, including both IAB and TACE, after the diagnosis of PVI.
Statistics
Statistical analysis was performed with SPSS. A two-tailed Student’s t-test or Wilcoxon rank-sum test was used for univariate analysis depending on data distribution. Unadjusted survival analysis was performed using a Kaplan-Meier curve and calculated from the date PVI was noted on imaging. A univariate and multivariate regression analysis was performed on the sub-group of liver-directed therapy patients with dichotomized variables.
Results
Patient characteristics (Table 2)
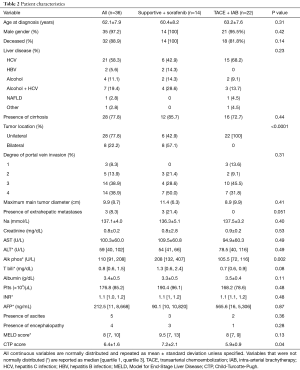
Full table
Thirty-seven HCC patients with PVI were identified. One patient was preoperatively diagnosed with cholangiocarcinoma but found to have HCC after resection, and was therefore excluded from further analyses. The remaining 36 patients included 35 men and 1 woman. There were no differences in age at diagnosis, etiology of liver disease, or presence or absence of cirrhosis between the systemic and liver-directed therapy groups. The majority of patients (59%) were diagnosed with PVI at the time of the initial HCC diagnosis; the remainder of patients had a mean time from HCC diagnosis to PVI diagnosis of 7.1±7.0 months. There was a significant difference between the groups in unilateral versus bilateral liver involvement; no patients with bilateral disease underwent liver-directed therapy during the study period. There were no statistically significant differences in degree of PVI or the presence of extrahepatic metastases at the time of diagnosis, although none of the three patients with extrahepatic metastases underwent liver-directed therapy. Alkaline phosphatase and Child-Turcotte-Pugh (CTP) score were significantly higher in the systemic therapy group than the liver-directed therapy group. There was a nonsignificant trend towards higher total bilirubin and Model for End-Stage Liver Disease (MELD) score in the systemic therapy group.
Treatment characteristics (Table 3)
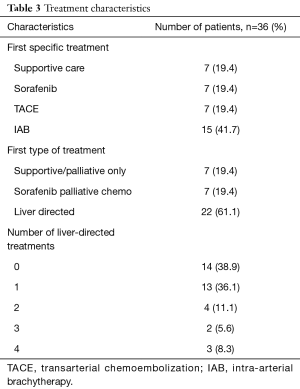
Full table
Of the 36 patients, seven were referred for supportive care, seven were recommended for treatment with sorafenib, and 22 underwent liver-directed treatment, including TACE in seven patients and IAB in 15 patients. Thirteen of the 22 patients treated with liver-directed therapy only underwent one treatment, while nine underwent more than one treatment. Two patients had complications: a 65-year-old man was admitted to the hospital for four days following TACE for altered mental status attributed to encephalopathy (without laboratory abnormalities) which improved with lactulose, and a 63-year-old man developed tachycardia within hours of TACE, was found to have an upper gastrointestinal bleed from a Mallory-Weiss tear, and required three days of hospitalization without the need for blood product transfusion. No patients had acute liver failure or death within 30 days of treatment, and no liver-directed therapy patients were readmitted to our institution at any time with acute liver failure.
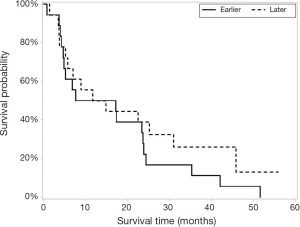
The median date of the diagnosis of PVI was identified, and further analysis was performed after divided the patients into “early” (diagnosed between 10/1/2009 and 8/8/2012) and “late” (diagnosed between 8/9/2012 and 6/30/2015) diagnosis groups. Equal number of patients in each group underwent liver-directed (n=11) and supportive care or sorafenib (n=7) during the early and late time periods.
Survival (Figure 2)

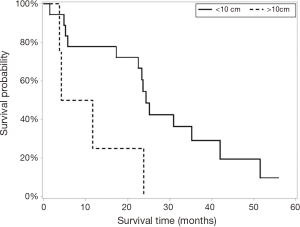
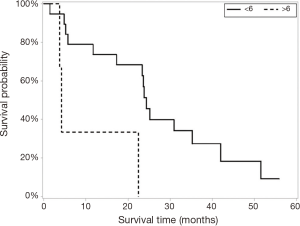
The liver-directed therapy group (n=22) had significantly longer median survival than the sorafenib/supportive care group (n=14) [23.6 (5.8, 30.9) vs. 6.0 (3.5, 8.8) months]. Maximum tumor diameter and CTP score were significantly different in univariate analysis (Figures 3 and 4) but only CTP was significantly different in multivariate modeling. Controlling for CTP in multivariate analysis, the survival benefit with liver-directed therapy remained significant when CTP was <6. There were no survival differences in age at diagnosis, degree of PVI, MELD score, or number of liver-directed therapies performed. Patients who received palliative care, supportive therapy, or no additional therapy (n=7) had significantly decreased survival compared to patients who received systemic chemotherapy with sorafenib (n=7) [4.7 (0.7, 6.8) vs. 8.8 (3.5, 17.1) months, P=0.038], although these results must be interpreted cautiously given the low number of patients in each group and wide confidence intervals. There were no significant differences in survival between patients in the early and late diagnosis groups who underwent liver-directed therapy (Figure 5).
Discussion
In a carefully selected group of patients with HCC with PVI treated TACE and IAB, median survival was significantly greater than the median survival in patients referred for sorafenib or palliative/supportive care. No liver-directed therapy patient experienced compromised liver function. Based on this data, patients with unilateral disease, low alkaline phosphatase, and low CTP score should be considered for liver-directed therapy. This patient population tolerated liver-directed therapy extremely well with only two minor complications, and no episodes of acute liver failure, other conditions requiring a prolonged hospitalization, or treatment related mortality. Expanding eligibility for liver-directed therapy to include patients with bilateral disease is not addressed by this data, but has the potential for efficacious treatment of more patients at the risk of increasing treatment-related complications. In the time since the study period, our institution has treated patients with bilateral HCC and PVI with liver-directed therapies in a staged manner to reduce the risk of hepatic failure, and this is an area ripe for future research. Even with the guidance of a standardized algorithm, the multi-disciplinary approach with involvement of hepatologists, medical oncologists, interventional radiologists, body imaging radiologists, surgical oncologists, liver transplant and hepatobiliary surgeons, palliative care specialists, social workers, a nurse coordinator, and a database manager is crucial for making the optimum treatment recommendations for each individual patient.
The results of the current study are in contrast with the traditional treatment plan for patients with PVI which is sorafenib or palliative care (16-18). This algorithm is limited by not accounting for performance status or underlying cirrhosis, and may be unnecessarily eliminating treatment options for patients with adequate hepatic reserve who could have a survival benefit with more aggressive treatment. Between 2000 and 2010, many studies in predominantly Asian populations described safe and efficacious resection combined with TACE, hepatic arterial infusion of chemotherapy, radiation, and ablative therapy as well as surgical resection alone in patients with HCC and Vp4 PVI (14). There is very limited data on the safety of resection in Western populations with HCC with PVI.
A Korean study on liver-directed therapy without resection in patients with HCC and PVI demonstrated comparable survival outcomes between sorafenib (n=31) and radioembolization (n=32), with fewer adverse effects in the radioembolization group (19). Similarly, a meta-analysis of eight Asian studies comparing TACE to conservative treatment showed an overall survival advantage at six months and one year in the TACE group, even in patients with main portal vein involvement. This report did note a wide range of transient liver decompensation (26–85%), but less than 2% incidence of acute liver failure (12).
Western groups have used IAB as a primary technique to treat patients with PVI because the embolization process is non-occlusive and therefore less likely to induce acute liver failure than TACE. Kulik et al. (7) treated 108 HCC patients with IAB, including 37 patients with PVI, and found that survival varied with the presence of cirrhosis and the extent of PVI. Patients with portal vein branch involvement had a median survival of 10 months while those with main portal vein involvement had a median survival of 4.4 months. Although the treatment was well tolerated in this high risk group, 58% of individuals with main vein involvement developed an adverse biliary event following treatment. Similar findings were reported by a Spanish group (20) who demonstrated a median survival time of 10 months after IAB treatment in 25 patients with HCC with PVI, including 14 patients with bilobar disease. The much greater 24-month median survival that we have demonstrated may be related to patient selection as well as technical refinements in IAB.
An American study (21) of 141 patients with locally aggressive HCC including 31 patients with portal vein thrombosis treated with TACE/DEB (transarterial chemotherapy with drug-eluting beads) or IAB demonstrated complete radiologic response in 35.9% of patients. This was associated with a significant survival advantage compared to patients with a partial response. They found overall tolerance of repeated liver-directed treatments without increased toxicity in this population, but noted a significant increase in adverse events six months after the final liver-directed treatment in patients with portal vein thrombosis at baseline, suggesting this subset of patients requires additional scrutiny.
In Southeast Asia, hepatitis B infection (HBV) is endemic and has historically been responsible for the majority of HCC cases while in the United States hepatitis C infection (HCV) and alcohol use are the predominant risk factors (22). Our study population is consistent with these trends with 58.3% of patients having HCV, 11.1% alcohol-related liver disease, and 19.4% HCV and alcohol-related versus only 5.6% of patients having HBV. The liver inflammation associated with HCV is more severe than HBV, which affects the safety of surgical resection and liver-directed therapy in these patients. National organization treatment guidelines reflect these population differences (4,5,23-26). These historical norms may be changing; a recent Japanese report found 53.3% of patients with HCV infection and only 23.3% with HBV infection (27). Unfortunately the recent major European and American studies of treatment of HCC with PVI do not differentiate between HBV and HCV infection (11,13,21).
The limitations of the present data are a small number of patients which precluded complex statistical analysis, as well as heterogeneity within the patient population and use of systemic therapies which may limit broad conclusions regarding decision making. There was also a lack of ability to standardize performance status and quality of life data collected, particularly in the patients treated with liver directed therapies. Although CTP score was significantly different between the groups, MELD did not differ between these two groups. These limitations may account for the failure to reproduce the previous findings of survival differences based on degree of PVI, MELD, and number of liver-directed therapies (9,10). Despite these factors, this study demonstrates that a carefully selected subset of patients with HCC and PVI have extended survival when treated with liver-directed therapy. There is an implicit selection bias in the comparison of groups of patients undergoing TACE or IAB versus systemic chemotherapy or palliative care, and a logical correlation with a survival difference between these two groups of patients.
The goal of this study was therefore not to explicitly compare survival between these heterogenous groups but to identify variables which could provide objective guidance for treatment decisions for future patients in the setting of existing literature from other parts of the world including survival benefits for patients treated with liver-directed therapies compared to traditional systemic chemotherapy. This personalized approach allows patients with poor prognostic factors to avoid the risks associated with invasive treatment modalities and maximize quality of life after diagnosis, with the added benefit of optimizing healthcare utilization by avoiding expensive, resource-intensive treatments in patients who are unlikely to benefit from these interventions.
Conclusions
These results demonstrate that patients with complex, advanced HCC can have extended survival and a low rate of adverse events with liver-directed therapy while high risk patients should be treated with sorafenib or in some cases, supportive care. Our results suggest that impending biliary obstruction is a useful clinical indicator of high risk disease but additional research is needed to further delineate criteria indicative of poor prognosis to allow for appropriate palliation and avoidance of interventions that are unlikely to provide a survival or quality of life benefit. The input of a multidisciplinary group of clinicians is essential for making these difficult, individualized treatment recommendations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board at Oregon Health & Sciences University (No. 16353) and written informed consent was obtained from all patients.
References
- Altekruse SF, Henley SJ, Cucinelli JE, et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol 2014;109:542-53. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Obi S, Yoshida H, Toune R, et al. Combination therapy of intraarterial 5- fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer 2006;106:1990-7. [Crossref] [PubMed]
- Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 2016;150:835-53. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang TK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47:71-81. [Crossref] [PubMed]
- Min YW, Kim J, Kim S, et al. Risk factors and a predictive model for acute hepatic failure after transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. Liver Int 2013;33:197-202. [Crossref] [PubMed]
- Chung GE, Lee JH, Kim HY, et al. Transarterial chemoembolization can be safely performed in patients with hepatocellular carcinoma invading the main portal vein and may improve the overall survival. Radiology 2011;258:627-34. [Crossref] [PubMed]
- Luo J, Guo RP, Lai EC, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol 2011;18:413-20. [Crossref] [PubMed]
- Pinter M, Hucke F, Graziadei I, et al. Advanced-stage hepatocellular carcinoma: transarterial chemoembolization versus sorafenib. Radiology 2012;263:590-9. [Crossref] [PubMed]
- Xue TC, Xie XY, Zhang L, et al. Transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombus: A meta-analysis. BMC Gastroenterol 2013;13:60. [Crossref] [PubMed]
- de la Torre MA, Buades-Mateu J, de la Rosa PA. A Comparison of survival in patients with hepatocellular carcinoma and portal vein invasion treated by radioembolization or sorafenib. Liver Int 2016;36:1206-12. [Crossref] [PubMed]
- Katagiri S, Yamamoto M. Multidisciplinary treatments for hepatocellular carcinoma with major portal vein tumor thrombus. Surg Today 2014;44:219-26. [Crossref] [PubMed]
- Yu SJ, Kim YJ. Effective treatment strategies other than sorafenib for the patients with advanced hepatocellular carcinoma invading portal vein. World J Hepatol 2015;7:1553-61. [Crossref] [PubMed]
- Fonseca AL, Cha CH. Hepatocellular carcinoma: A comprehensive overview of surgical therapy. J Surg Oncol 2014;110:712-9. [Crossref] [PubMed]
- Graf D, Vallböhmer D, Knoefel WT, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med 2014;25:430-7. [Crossref] [PubMed]
- Galun D, Basaric D, Zuvela M, et al. Hepatocellular carcinoma: From clinical practice to evidence-based treatment protocols. World J Hepatol 2015;7:2274-91. [Crossref] [PubMed]
- Cho YY, Lee M, Kim HC, et al. Radioembolization is a safe and effective treatment for hepatocellular carcinoma with portal vein thrombosis: A propensity score analysis. PLoS One 2016;11:e0154986. [Crossref] [PubMed]
- Iñarrairaegui M, Thurston KG, Bilbao JI, et al. Radioembolization with use of Yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2010;21:1205-12. [Crossref] [PubMed]
- Newell PH, Wu YX, Hoen H, et al. Multimodal treatment of unresectable hepatocellular carcinoma to achieve complete response results in improved survival. HPB 2015;17:454-60. [Crossref] [PubMed]
- Zhu AX. Current status of hepatocellular carcinoma in the United States. Chin Clin Oncol 2013;2:45.
- Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer, Second English Edition. Tokyo: Kanehara & Co., Ltd., 2003.
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74. [Crossref] [PubMed]
- European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Sun JX, Li N, Guo WX, et al. Portal vein tumor thrombus is a bottleneck in the treatment of hepatocellular carcinoma. Cancer Biol Med 2016;13:452-8. [Crossref] [PubMed]
- Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016;65:938-43. [Crossref] [PubMed]