The influence of radiation therapy dose escalation on overall survival in unresectable pancreatic adenocarcinoma
Introduction
Pancreatic adenocarcinoma (PAC) remains a substantial cause of cancer mortality in the United States (1). Patients with non-metastatic, unresectable PAC constitute a large percentage of PAC patients and are a group in which aggressive local therapy could potentially improve outcomes (2). The use of preoperative chemotherapy has increased and aids in selection of those patients that would otherwise develop distant metastatic disease (3). Furthermore, a recent autopsy series demonstrated exciting genetic markers that can predict failure patterns along with evidence that close to one third of patients with unresectable PAC die from predominately local disease progression (4). These recent advancements are bringing us closer to selecting those patients with unresectable PAC that may truly benefit from aggressive local therapy.
A considerable number of clinical trials have been conducted examining RT dose escalation (5-14). These trials have resulted in conflicting conclusions regarding the benefits of increasing RT dose. The goal of this series was to examine the effect of RT dose escalation in non-metastatic, unresectable PAC through an analysis of the national cancer data base (NCDB).
Patients and methods
Our patient population was obtained from the Pancreatic Participant Use Data File (PUF) from the NCDB, which is one of the world’s largest clinical cancer registries (15). The NCDB is supported by the American College of Surgeons and the American Cancer Society (15) and includes more than 1,440 hospitals in the United States. Data available include patient demographics, pathologic characteristics, detailed staging, RT dose information, chemotherapy data, and overall survival (OS) data.
Emory University was granted alpha-test user site status for the Pancreatic PUF, which includes all incident cases of PAC reported to the NCDB for the 5-year period of 1998-2002. PUF’s are entirely de-identified data files available to selected investigators at Commission on Cancer (CoC) approved institutions for the advancement of patient care. Results reported are in compliance with the privacy requirements of the Health Insurance Portability and Accountability Act of 1996 as described in the Standards for Privacy of Individually Identifiable Health Information; Final Rule (45 CFR Parts 160 and 164). The use and publication of these data have been previously subject to peer review and approval by the NCDB.
There were 94,385 incident cases in the Pancreatic PUF for the 1998-2002 period. Of these, we selected patients with a primary tumor site in the pancreas resulting in 69,268 analyzable patients. We then selected 54,138 patients who did not have surgery on the primary site. From this group we selected 9,183 patients who underwent a documented course of external beam RT, thus excluding patients with missing information. Patients without evidence of distant metastatic disease were included, and pathologic M1 patients were excluded, leaving 7,044 patients. We then selected only those patients coded as having unresectable disease leaving 5,544 patients. Patients were then eliminated if they were coded as having T0, T1, or T2 disease leaving 4,532 patients. Any remaining patients coded as having stage I, or both an unknown T or group stage were also excluded leaving 4,023. Patients that did not receive chemotherapy were then excluded leaving 3,579. Patients were then selected that did not have missing survival information leaving 3,576. We then selected patients for whom the radiation dose was known leaving a total of 989 patients (coding radiation dose was optional until 2003). Finally, 12 patients with inaccurately coded RT doses (defined as any inconceivable dose of RT either less than 1 Gy or greater than 100 Gy) were eliminated leaving the final total of 977 patients. Among patients that met the first nine criteria, patients that met all criteria (n=977) vs. those that were excluded due to missing survival information, missing radiation dose, or incorrect dose were compared. Differences were assessed using chi-square test or analysis of variance.
Covariates included age, gender, race, facility type, facility volume, radiation dose, radiation duration, stage, tumor size, and grade. Facility volume was calculated as the total number of PAC cases in a given facility during the years 1998-2002. Facility types were designated as Community Cancer Programs (CCP), Comprehensive Community Cancer Programs (CCCP), or Academic/Research Programs (ARCP). The primary outcome was OS, and if a patient survived beyond 60 months, OS was censored at 61 months. Initially dose was examined as a continuous variable and also dichotomized based on the median dose. Categories for radiation dose were then chosen based on martingale residual plots, and were then further adapted to be based on clinically meaningful dose levels determined by a consensus group of the authors.
Statistical analysis was conducted using SAS Version 9.3. The significance level was set at 0.05. Descriptive statistics for each variable were reported. The unadjusted association of each variable with OS was derived from a Cox proportional hazards model. The chi-square test was used for categorical covariates and analysis of variance was used for numerical covariates to compare the covariates across the different radiation dose levels. Kaplan-Meier method was used to generate OS curves and estimate median survival with 95% confidence intervals. Radiation duration and tumor size were excluded from all multivariate (MV) analysis due to a high number of missing values. The MV survival analysis included dose, stage, facility type, and facility volume. The other covariates were entered in the model subject to a backward variable selection method with an alpha =0.05 removal criteria.
Propensity scores were calculated using a nominal logistic regression model to predict radiation dose. Inverse probability of treatment weights (IPTW) were calculated and represented the inverse probability of a participant receiving the observed dose based on their characteristics. IPTW estimates were further stabilized by multiplying them by the marginal probability of receiving the observed dose. The multivariable survival analysis was repeated, weighting by the stabilized IPTW. Weights were normalized to add up to the original sample size.
Results
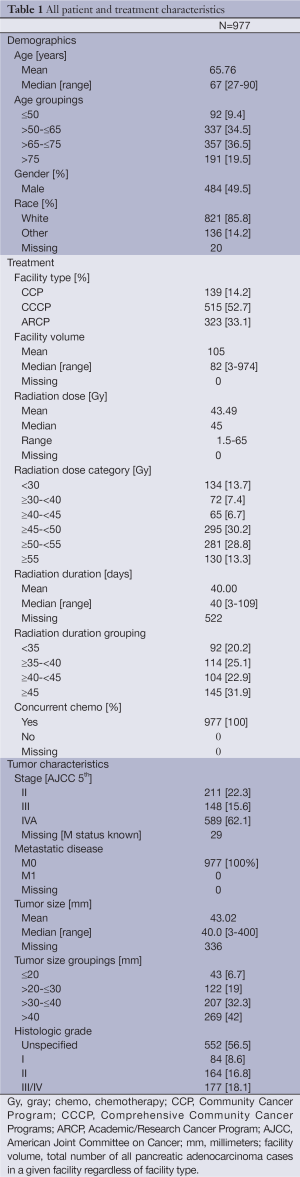
A total of 977 analyzable patients were identified during the time interval assessed meeting inclusion criteria. There were no significant differences in patient characteristics, other than facility type and volume, between excluded patients and those presented. Median age was 67 years (range, 27-90 years), 49.5% were male, and 85.8% were Caucasian. All patients were treated with RT and chemotherapy. The staging was 5th edition American Joint Committee on Cancer (AJCC) staging and consisted of 211 AJCC stage II, 148 stage III, 589 stage IVA, and 29 patients had missing stage information. Median tumor size was 4.0 cm (range, 0.3-40 cm) and all patients were negative for distant metastatic disease (M0). Median RT dose was 45 Gy (range, 1.5-65 Gy), and median treatment duration was 40 days (range, 3-109 days). 134 patients (13.7%) received <30 Gy, 72 (7.4%) received ≥30 to <40 Gy, 65 patients (6.7%) received ≥40 Gy to <45 Gy, 295 (30.2%) received ≥45 Gy to <50 Gy, 281 (28.8%) received ≥50 to <55 Gy, and 130 (13.3%) received ≥55 Gy. A detailed summary of patient and treatment characteristics is found in Table 1.
Full table
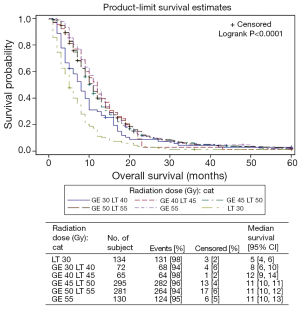
The median OS for patients receiving less than 30 Gy was five months (95% CI, 4-6 months); for those patients receiving between ≥30 to <40 Gy was 8 months (95% CI, 6-10 months); for those receiving ≥40 to <45 Gy median OS was 12 months (95% CI, 9-14 months); for those receiving ≥45 to <50 Gy median OS was also 11 months (95% CI, 10-11 months); for those receiving ≥50 to <55 Gy median OS was also 11 months (95% CI, 10-12 months) and for those receiving greater than 55 Gy median OS was 11 months (95% CI, 10-13 months). The KM OS analysis for each dose level is shown in Figure 1.
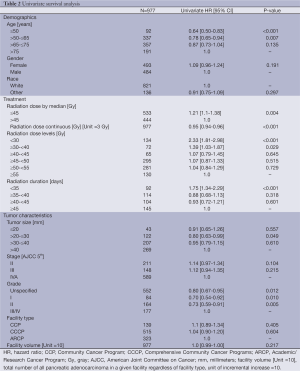
In the UV survival analysis, several different adjuvant treatment parameters were associated with higher risk of death including RT dose below the median, RT dose <30 Gy, and RT dose ≥30 to <40 Gy, and shorter radiation duration. Factors significantly associated with lower risk of death included, smaller tumor size, lower grade, and younger age. The results of the complete UV can be found in Table 2.
Full table
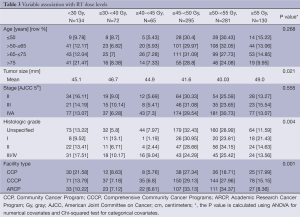
The UV associated between categorized radiation dose and all other covariates are summarized in Table 3. Factors found to be significantly correlated with the different dose level categories of RT included facility type, tumor size, and grade. It can be seen that the RT dose was independent of stage.
Full table
In the MV survival analysis, RT dose <30 Gy [HR, 2.38 (95% CI, 1.85-3.07); P≤0.001] and RT dose ≥30 Gy and <40 Gy [HR, 1.41 (95% CI, 1.04-1.91); P=0.026] vs. RT dose ≥55 Gy; were significantly associated with worse OS. In addition to radiation dose, age was also found to be significant on MV analysis. The complete MV survival analysis can be seen in Table 4. As the results of the MV survival analysis were not significantly different with and without the propensity score weighting, we present the unweighted results only.

Full table
The duration of time over which each of the respective RT doses was delivered is summarized in Table 5. It can be seen that the vast majority of patients for which the RT duration was known received conventionally fractionated RT.
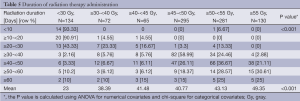
Full table
Discussion
The purpose of this analysis of the NCDB was to examine the effect of RT dose escalation in a large cohort of patients with unresectable PAC. This series presents a heterogeneous group of patients, treated in a variety of facility types, with a wide range of RT doses. There was no measureable benefit or detriment to OS in patients treated with conventionally delivered, escalating RT doses greater than 40 Gy.
There exists a historical precedent for RT dose escalation in unresectable PAC. An early prospective study examining RT dose escalation was the Gastrointestinal Tumor Study Group’s (GITSG) locally advanced dose escalation trial (5). Published in 1981, this prospective trial randomized 194 patients to 60 Gy of RT alone or concurrent chemo-RT with dose escalated RT consisting of 40 vs. 60 Gy. The RT was delivered over a split course using a two week intervening break with concurrent 5-FU based chemotherapy. A significant benefit was demonstrated with the addition of chemotherapy to RT, but no benefit was seen with RT dose escalation. The median OS for patients in the moderate high dose chemo-RT arms were both approximately ten months (5).
Profound technical advances in RT delivery have inspired an array of modern RT dose escalation series in unresectable PAC using a variety of RT delivery methods (6,8,10-12,14). In some series median OS has remained comparable to that demonstrated by the GITSG trial nearly 25 years prior (8,14). The heterogeneous results from these trials have resulted in conflicting conclusions regarding the benefit of radiosurgical dose escalation, with some series concluding that radiosurgical boost has no role in dose escalation for unresectable PAC (14). Still, more recent series have concluded that this technology is promising and warrants further investigation (6,8,9). The question remains, despite the improvements in local control seen with dose escalation, what additional factors associated with these dose escalation trials could be contributing to only a minimal change in OS numbers? The most likely explanation is that patients treated with dose escalation have increased toxicity detrimental to OS or that poorly selected patients succumb to subsequent distant metastatic disease.
There is room for tremendous speculation as to why RT dose escalation has failed thus far in unresectable PAC. As with any aggressive local therapy, patient selection remains absolutely critical. The ability to select those patients that will not fail distantly after completing a course of aggressive local therapy is essential to translating local control improvements into meaningful OS improvements. Recently, great advancements in patient selection through both neoadjuvant chemotherapy and genetic analysis have provided hope in this arena (3,4,16). Additionally, an often overlooked and understudied area of RT delivery in unresectable PAC is the modality of GTV delineation. Recently, retrospective data have emerged and called into question the volumes delineated on abdominal CT and MRI (17,18). When local tumors are treated alone with increasingly small margins, the process of a pancreatic tumor GTV delineation must be carefully studied before a minimal margin is used expanding GTV-PTV. The GTV delineation in this disease may have important implications for normal tissue toxicity and local control, particularly in the setting of dose escalation.
Despite the conflicting trials, hope remains for improved outcomes with RT dose escalation in unresectable PAC. In a series by Ben-Josef et al., high quality intensity modulated radiation therapy (IMRT) with strict dose constraints was delivered in a Time-to-Event Continual Reassessment (TITE-CRM) trial that accrued a total of 50 patients (19). The recommended dose was determined to be 55 Gy over 25 fractions, and 2-year OS was an encouraging 14.8 months (11). A combination of rigorous patient selection, meticulous RT dose constraints, improved gemcitabine delivery, and prospective RT quality assurance likely contributed to the improvement in outcomes demonstrated in the Ben-Josef et al. series (11).
Using retrospective data analysis of a large cohort of patients from the NCDB, the current series demonstrates the absence of an OS benefit or detriment with RT dose escalation above 40 Gy. Our results agree with those of past randomized trials, which offer little evidence that conventionally fractionated 3D conformal RT (3D-CRT) delivery above 40 Gy improves patient outcomes in unresectable PAC. Recent American-French consensus guidelines have supported a dose range of 50-54 Gy, which is primarily based on the dose used in published randomized trials (20).
Another potentially important interpretation of the current series is that while there was no measureable benefit to RT dose escalation, there was also no detriment shown. Recent Phase III data have emerged that have demonstrated a detriment to OS with the addition of high dose chemo-RT as compared with chemotherapy alone (21). This has led to the conclusions that chemotherapy alone should be used in patients with unresectable PAC over chemo-RT, which is primarily practiced in Europe. Our series presents a large cohort of patients, treated in a variety of facility types, with escalating RT doses to 65 Gy without any measureable detriment to OS with increasing RT dose. If such a detriment to OS existed secondary to RT toxicity, one may expect to see it manifest in this large cohort of patients with increasing RT doses.
There are considerable limitations to any retrospective series and any large centralized database analysis. Such limitations include errors in data coding, absence of precise chemotherapy details, unknown CA-19-9 levels, lack of specific failure patterns, unknown medical comorbidities, and unknown performance status. Furthermore, a relatively small percentage of all available patients are included in this analysis, which introduces a potential confounder. We have conducted additional analysis to attempt to control for selection bias, including an analysis of all excluded patients and a propensity score adjusted analysis. These additional analyses had no influence on the conclusions drawn in the manuscript. Moreover, depending on the chemotherapy used differences might exist between the biological effectiveness of the RT dose levels we have examined. While we expect that given the treatment dates of 1998-2002 the majority of these patients received concurrent 5-FU based chemotherapy, the precise type and dose of chemotherapy is not known. Additionally the use of split course radiation is not known with certainty, and while it appears the majority of patients received conventional fractionation based on Table 5, however, we cannot be certain with the RT data included in the NCDB. Finally, certain dose levels compared have relatively small patient numbers, such as the 40 to <45 Gy cohort. This makes firm conclusions as to the optimal dose level difficult to ascertain from this analysis.
Limitations notwithstanding, these NCDB data offer a number of highly unique strengths. At the time of submission, the analysis is the largest conducted specifically examining RT dose escalation in unresectable PAC. The number of patients, knowledge of RT dose, chemotherapy, detailed staging, and diversity of facility types, provides insight into the outcomes of dose escalation across a wide range of practice settings. Such an analysis would be difficult without a large centralized database design.
The true role of RT dose escalation remains unknown in unresectable PAC. As the sequencing of chemotherapy and RT shift to preoperative delivery the potential benefits of preoperative RT dose escalation will require additional examination and have shown promise in a recent meta-analysis (22,23). Furthermore, the ability of dose escalation to convert previously unresectable patients to resectable is exciting and was demonstrated in the series by Ben-Josef et al. (11). Overall, it is becoming abundantly clear that the delivery of dose escalated RT in unresectable PAC should take place in the setting of meticulously designed, prospective clinical trials with a substantial focus on RT quality, multidisciplinary assessment, and rigorous patient selection.
Acknowledgements
Grant support: This work was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 and TL1TR000456. Research reported in this publication was supported in part by the Biostatistics & Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. We would like to thank the American College of Surgeons Commission on Cancer for access to the data that enabled this analysis.
Disclosure: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg 1993;165:68-72; discussion 72-3. [PubMed]
- Krishnan S, Rana V, Janjan NA, et al. Induction chemotherapy selects patients with locally advanced, unresectable pancreatic cancer for optimal benefit from consolidative chemoradiation therapy. Cancer 2007;110:47-55. [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [PubMed]
- Moertel CG, Frytak S, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The Gastrointestinal Tumor Study Group. Cancer 1981;48:1705-10. [PubMed]
- Chang DT, Schellenberg D, Shen J, et al. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer 2009;115:665-72. [PubMed]
- Ko AH, Quivey JM, Venook AP, et al. A phase II study of fixed-dose rate gemcitabine plus low-dose cisplatin followed by consolidative chemoradiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2007;68:809-16. [PubMed]
- Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;58:1017-21. [PubMed]
- Mahadevan A, Miksad R, Goldstein M, et al. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys 2011;81:e615-22. [PubMed]
- Schellenberg D, Kim J, Christman-Skieller C, et al. Single-fraction stereotactic body radiation therapy and sequential gemcitabine for the treatment of locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 2011;81:181-8. [PubMed]
- Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2012;84:1166-71. [PubMed]
- Ceha HM, van Tienhoven G, Gouma DJ, et al. Feasibility and efficacy of high dose conformal radiotherapy for patients with locally advanced pancreatic carcinoma. Cancer 2000;89:2222-9. [PubMed]
- McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2001;19:4202-8. [PubMed]
- Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 2005;76:48-53. [PubMed]
- Winchester DP, Stewart AK, Bura C, et al. The National Cancer Data Base: a clinical surveillance and quality improvement tool. J Surg Oncol 2004;85:1-3. [PubMed]
- Huguet F, André T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326-31. [PubMed]
- Arvold ND, Niemierko A, Mamon HJ, et al. Pancreatic cancer tumor size on CT scan versus pathologic specimen: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys 2011;80:1383-90. [PubMed]
- Hall WA, Mikell JL, Mittal P, et al. Tumor size on abdominal MRI versus pathologic specimen in resected pancreatic adenocarcinoma: implications for radiation treatment planning. Int J Radiat Oncol Biol Phys 2013;86:102-7. [PubMed]
- Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys 2004;59:454-9. [PubMed]
- Huguet F, Goodman KA, Azria D, et al. Radiotherapy technical considerations in the management of locally advanced pancreatic cancer: American-French consensus recommendations. Int J Radiat Oncol Biol Phys 2012;83:1355-64. [PubMed]
- Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol 2008;19:1592-9. [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [PubMed]
- Colbert LE, Hall WA, Nickleach D, et al. Chemoradiation therapy sequencing for resected pancreatic adenocarcinoma in the National Cancer Data Base. Cancer 2014;120:499-506. [PubMed]