Overall survival analysis of neoadjuvant chemoradiotherapy and esophagectomy for esophageal cancer
Introduction
In the United States alone nearly 18,000 new esophageal cancers are diagnosed and more than 15,000 deaths occur each year, illustrating the high mortality of this disease and the ongoing need for improved treatment strategies (1). Randomized controlled trials comparing neoadjuvant chemoradiotherapy (NAC) with surgery alone have demonstrated statistically significant improvements in overall survival (OS) (2-5). More recently, the CROSS trial modified traditional chemotherapy protocols, introducing weekly administration of carboplatin and paclitaxel with concomitant radiotherapy. This resulted in a clear OS benefit for NAC versus surgery alone, with a median OS of 49.3 versus 24 months, respectively (5). These studies are consistent with several meta-analyses, which demonstrate that NAC significantly increases OS compared to surgery alone (6-9). Taken together, these studies highlight the utility of NAC in the treatment of esophageal cancer.
In addition to providing a clear survival benefit, NAC increases the likelihood of an R0 resection (6), which is associated with significantly improved OS in patients with esophageal cancer (10). Importantly, the pathologic stage following esophagectomy in patients treated with NAC is a strong predictor of OS, and in particular, downstaging by NAC is associated with improved disease-free survival (DFS) and OS (11). Additional studies have demonstrated that patients with a pathologic complete response (pCR) following NAC and esophagectomy have high long-term OS rates (12,13).
Based on these and other data, multimodality treatment including NAC followed by esophagectomy has been established as standard of care for early stage (II-III), resectable esophageal cancer and that patients treated with NAC are more likely to have an R0 resection and pCR, more likely to be downstaged, and have improved DFS and OS. Therefore, the specific aim of the current study was to analyze OS outcomes of NAC at a single, tertiary care academic medical center. Additional objectives were to quantify the downstaging secondary to NAC, analyze the impact of downstaging on OS, and determine to what extent histologic subtype affects OS.
Materials and methods
Eligibility
The study was approved by the Institutional Review Board at Oregon Health & Science University (OHSU) and patient informed consent was waived. Medical records from patients with esophageal malignancies treated with NAC followed by esophagectomy at OHSU from September 1996 to May 2011 were selected from a prospective esophageal registry and retrospectively reviewed. Eligible patients included those with stage I-III esophageal cancer deemed medically operable by an experienced general or thoracic surgeon or medically inoperable who went on to receive NAC and were subsequently deemed operable. Patients with recurrent or metastatic disease, a history of previous malignancy, and as those unable to undergo chemoradiotherapy were excluded from the study. A cohort of 106 consecutive patients formed the basis of this selection.
Treatment plans
Patients who underwent NAC were treated with platinum-based chemotherapy (including cisplatin, oxaliplatin, or carboplatin) together with 5-fluorouracil (5-FU) or capecitabine concurrently with radiation. Additionally, 17 patients received a mitotic inhibitor (paclitaxel or docetaxel) in their regimen. Notable exceptions include six patients who received platinum-based therapy but did not receive 5-FU or capecitabine and a single patient who received paclitaxel and 5-FU but did not tolerate a platinum-based agent. The majority of patients received 50.4 Gy radiation by standard fractionation, although cumulative dose ranged from 36-63 Gy. Surgical resection was performed via a transhiatal, Ivor-Lewis, or 3-field approach as previously described (14,15). Eligible patients underwent chemoradiotherapy at OHSU as well as local community hospitals, however all surgical resections were performed at OHSU by experienced general, thoracic, and/or oncologic surgeons.
Staging and pathology
Prior to administering NAC, all patients were staged by endoscopic ultrasound (EUS), computed tomography (CT), or positron emission tomography (PET). Following NAC and esophagectomy, post-operative pathological staging was compared to initial staging to analyze the effect of NAC and subsequent down- or upstaging. In this study, an R0 resection is defined as a curative resection, with microscopic examination of margins demonstrating absence of tumor cells while a R1 resection demonstrates the presence of tumors cells at the margin of resection. A pCR is defined as the absence of any residual tumor cells during histologic examination.
Survival analysis and statistical methods
Clinical follow up and the Social Security Death Index were used to determine length of survival for each patient. OS was analyzed by the Kaplan Meier method and survival curves were generated using R statistical software (version 2.13.1, R Development Core Team, Vienna, Austria). Inter-group comparisons were analyzed using a log-rank test and statistical significance was determined by P value <0.05.
Results
Patient and tumor characteristics
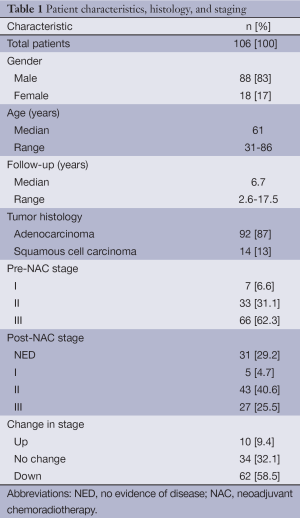
We analyzed 106 patients with esophageal cancer that underwent NAC followed by esophagectomy from September 1996 to May 2011. Patient characteristics as well as tumor histology and staging are presented in Table 1. The vast majority of patients in this study were male (n=88, 83%) and the median age was 61 (range, 31-86) years at the time of diagnosis. The predominant histology was adenocarcinoma (n=92, 87%) while 13% were squamous cell carcinoma (n=14). Prior to treatment, nearly two-thirds of patients presented with stage III disease (n=66, 62%), with stage IIIA being the most frequent presenting stage (n=51, 48%), while one-third had stage II (n=33, 31%) and 7% had stage I (n=7) disease. Median follow up was 6.7 (range, 2.6-17.5) years.
Full table
Pathologic response and post-operative staging
Following NAC and esophagectomy, a pCR with no evidence of disease histologically was achieved in 31 patients (29%) of the cohort. Moreover, the majority of patients had an R0 resection with negative margins microscopically (n=98, 92.5%). Grossly, 14 patients (13.2%) had an R1 resection with confirmed positive margins in 8 patients (7.5%). Expectedly, post-operative pathologic staging determined that 62 patients (59%) were downstaged following NAC while 9 patients (8%) were upstaged and 34 patients (32%) remained at the same stage (Table 1).
Survival analysis
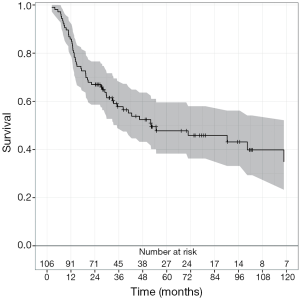
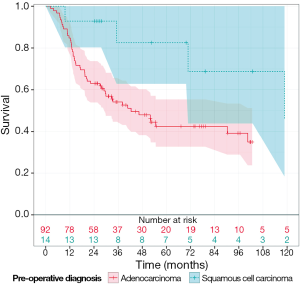
The median OS was 31.2 months (range, 2 months -17 years) for all patients in this cohort (Figure 1). When analyzed by histologic subtype, there was a trend toward increased OS in patients with squamous cell carcinoma vs. adenocarcinoma (53 vs. 29 months, respectively; P=0.06, Figure 2). Interestingly there was a similar extent of downstaging between squamous cell carcinomas and adenocarcinomas (50% vs. 51.9%, respectively). However 35.7% (n=5 of 14) of squamous cell carcinomas had a pCR compared to only 24.5% (n=23 of 92) of adenocarcinomas. Moreover, there were a greater proportion of patients who had squamous cell carcinoma with stage III disease compared to those in the adenocarcinoma group (78.6% vs. 51.9%, respectively).
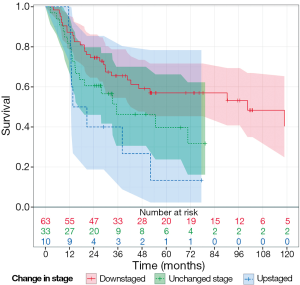
Importantly, there was also a trend toward increased OS for downstaged patients following NAC and esophagectomy (P=0.08, Figure 3).The OS for downstaged patients was 40 months, upstaged patients was 20.6 months, and 27 months for those who remained at the same stage. Patients that had no evidence of disease on histological exam at surgery (pCR) had a median OS of 52 months.
Discussion
The results in this study demonstrate that NAC was effective in downstaging the majority of patients in this cohort and effectively increased the chance of an R0 resection. These findings are important, as pathologic stage following esophagectomy in patients treated with NAC is a strong predictor of OS. Consequently, downstaging by NAC is associated with improved DFS and OS (11). The patients in this study had improved OS survival compared to the median OS, suggesting patients from this tertiary care academic medical center treated with NAC and esophagectomy had similar outcomes compared to those in recent multi-center clinical trials (5,13). Additional studies have demonstrated that patients with a pCR following NAC and esophagectomy have high long-term OS rates (12,13). Our findings are consistent with these results and patients in our cohort that had a pathological complete response rate had a median OS of 52 months.
Interestingly, our patients with squamous cell carcinoma showed a trend toward more favorable OS compared to those with adenocarcinoma. The relationship between histologic subtype and OS in esophageal cancer is multifactorial and not completely understood at the present time. Indeed, studies in early stage esophageal cancer suggest squamous cell carcinomas are more susceptible to distant lymphatic spread and confer reduced 5-year OS rates (16). Conversely, analysis of patients with esophageal cancer and non-regional nodal metastasis revealed squamous cell histology was an independent positive predictor of long-term survival following esophagectomy (17). Given that the majority of patients in our cohort presented with stage III disease, our results are consistent with those studies in more advanced disease and suggest squamous cell histology confers a more favorable OS. However, as only 13% of patients in our study had squamous cell carcinoma, further characterization of the factors contributing to this observation is not possible within this current study.
While these results have contributed to the understanding of the effectiveness of NAC followed by esophagectomy for esophageal cancer at a single academic medical center, there are particular limitations of this study. Once such limitation was the variation in chemotherapy and radiation regimens used throughout the 15 years for which patients were analyzed in this cohort. These treatment alterations introduced additional variables difficult to account for given the heterogeneity of treatment plans and improvement of surgical techniques over such a lengthy time period. Additionally, while this study identified a trend in improved OS compared to the median OS for downstaged patients following NAC and esophagectomy, this study was underpowered to detect a statistically significant difference.
In conclusion, this study analyzed OS outcomes for patients with esophageal cancer who underwent NAC followed by esophagectomy at a single, tertiary care academic medical center. This study determined that NAC was effective in downstaging the majority of patients and effectively increased the chance for an R0 resection. These patients, in turn, had improved OS compared to the median OS. Together, the results of this study support the rationale for NAC followed by esophagectomy in effectively downstaging patients and increasing the likelihood of an R0 resection and improved OS.
Acknowledgements
Funding: KMA is a Rubinstein Radiation Research Scholar.
The authors would like to acknowledge the professionalism, expertise and dedication of the radiation therapists at the Oregon Health & Science University Knight Cancer Institute; Dr. Dolan’s authorship in this publication was supported by the Oregon Clinical and Translational Research Institute (OCTRI), and a grant (No. UL1TR000128) from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Disclosure: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [PubMed]
- Cao XF, He XT, Ji L, et al. Effects of neoadjuvant radiochemotherapy on pathological staging and prognosis for locally advanced esophageal squamous cell carcinoma. Dis Esophagus 2009;22:477-81. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Urschel JD, Vasan H. A meta-analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg 2003;185:538-43. [PubMed]
- Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta-analysis. Gut 2004;53:925-30. [PubMed]
- Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34. [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [PubMed]
- Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 2005;23:4330-7. [PubMed]
- Chirieac LR, Swisher SG, Ajani JA, et al. Posttherapy pathologic stage predicts survival in patients with esophageal carcinoma receiving preoperative chemoradiation. Cancer 2005;103:1347-55. [PubMed]
- Hammoud ZT, Kesler KA, Ferguson MK, et al. Survival outcomes of resected patients who demonstrate a pathologic complete response after neoadjuvant chemoradiation therapy for locally advanced esophageal cancer. Dis Esophagus 2006;19:69-72. [PubMed]
- Pasini F, de Manzoni G, Zanoni A, et al. Neoadjuvant therapy with weekly docetaxel and cisplatin, 5-fluorouracil continuous infusion, and concurrent radiotherapy in patients with locally advanced esophageal cancer produced a high percentage of long-lasting pathological complete response: a phase 2 study. Cancer 2013;119:939-45. [PubMed]
- Levy RM, Trivedi D, Luketich JD. Minimally invasive esophagectomy. Surg Clin North Am 2012;92:1265-85. [PubMed]
- Perry Y, Fernando HC. Three-field minimally invasive esophagectomy: current results and technique. J Thorac Cardiovasc Surg 2012;144:S63-6. [PubMed]
- Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 2005;242:566-73; discussion 573-5. [PubMed]
- Lee PC, Port JL, Paul S, et al. Predictors of long-term survival after resection of esophageal carcinoma with nonregional nodal metastases. Ann Thorac Surg 2009;88:186-92; discussion 192-3. [PubMed]