Analysis of surgical complications of primary tumor resection after neoadjuvant treatment in stage IV colon cancer
Introduction
Colorectal cancer (CRC) is considered the third most common cancer in men, and second in women worldwide (1). Surgical treatment is the most important approach in these patients, and in 80% of them, surgery can be performed. In locally advanced tumors, surgery must be supplemented with chemotherapy. Nowadays there is a progressive increase of synchronous metastatic CRC, and approximately 20% of patients present distant metastases at the time of diagnosis (2). In stage IV CRC, systemic chemotherapy is the cornerstone of therapy, taking into account that if a good response is obtained, surgical treatment could be performed. Between 40% and 70% of these patients will present a good response to palliative systemic treatment. The 5-year survival rate at stage IV is approximately 11.7% (3,4). About one half of non-metastatic CRC will develop liver metastases during follow-up. If metastases are restricted to liver or lung, and a complete excision of them can be achieved, these patients can benefit from curative surgery, with a significant benefit in overall survival (OS) (5,6).
There is a recent trend to treat preoperatively locally advanced colon cancer in order to let patients benefit from neoadjuvant chemotherapy, achieve downstaging, and diminish the recurrence rate (7,8). In this scenario it would be interesting to determine the surgical morbidity rates of colon surgery after neoadjuvant chemotherapy in other group of patients.
This study assesses the surgical complications of primary tumor resection in stage IV colon cancer patients—excluding rectal cancer—treated with preoperative chemotherapy. The physiological and operative severity score for the enumeration of mortality and morbidity (POSSUM) and the Portsmouth-POSSUM (P-POSSUM)—a modification of the POSSUM—scoring systems allow to compare the outcomes of surgical procedures according different degrees of complexity (9-11). They offer a way of estimating the probability of morbidity and mortality taking into account the magnitude of surgery and the preoperative physiological status of the patients. These scores are a good tool to evaluate if neaodjuvant therapy increases or not the expected complication rate.
Material and methods
This retrospective study included consecutive clinical stage IV colon adenocarcinoma patients who underwent primary tumour surgery after receiving preoperative chemotherapy at our center, between July 1st, 2001 and September 30th, 2010.
Study protocol, treatment scheme and follow-up
Initial clinical staging was performed through a colonoscopy with tumor biopsies, tumor markers and computed tomography (CT). In some cases diagnosis was supplemented with positron emission tomography (PET) or liver magnetic resonance image (MRI). Exclusion criteria included tumors within 15 cm of the anal verge, judged by sigmoidoscopy, or those below the level of the sacral promontory, judged by imaging methods. Patients with bleeding or obstruction underwent surgical treatment and they were not included in this study. Patients received different schemes of preoperative chemotherapy based on FOLFIRI (12), XELOXIRI (13) or XELOX plus cetuximab regimen (14).
Patients were re-staged at the end of chemotherapy and before surgery to value tumor response and determine surgical treatment feasibility. After completing chemotherapy, patients were subjected to radical excision of the primary tumor after four weeks from the end of chemotherapy. Occasionally, liver surgery was performed during the same surgical procedure.
Postoperative complications were defined as any clinical condition that required prolonged hospital stay or any deviation from the normal postoperative course. Operative mortality was stated as death within the first 30 days postoperative or during hospital admission after the surgical procedure. It was tried to minimize risk of infection, so maintenance of venous accesses or bladder catheters was valued on a daily basis. Furthermore, antithrombotic prophylaxis was accomplished by using low molecular weight heparin, pneumatic compression boots during the operation and compression stockings. Early ambulation and respiratory physiotherapy was encouraged. Nasogastric tube and drainage systems were reserved for selected cases, trying to restrict their usage.
Statistical methods
Patient data were obtained from the medical records. The incidence of complications was calculated for the entire population. Values are expressed as medians with range in quantitative variables, or as percentages of the group of origin in categorical variables. All statistical analyses were performed using SPSS software (version 15.0, SPSS Inc., Chicago, IL, USA).
Results
Between July 1, 2001 and September 30, 2010, 67 patients went through surgical excision of the primary tumour after receiving chemotherapy for stage IV colon cancer.
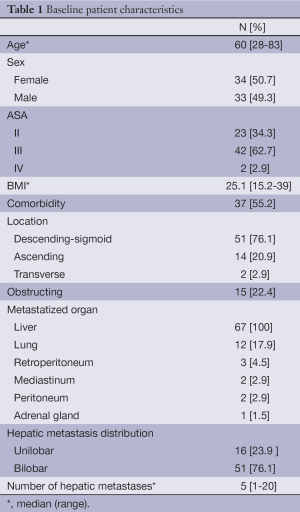
Table 1 shows baseline patient characteristics. All patients were affected with liver metastasis, with 76.1% (n=51) of them affecting both lobes. Furthermore, 29.8% (n=20) of them had metastasis in additional organs. The mean carcinoembryonic antigen (CEA) at the moment of initial diagnosis was 107.5 ng/mL.
Full table
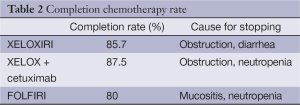
Twenty eight patients (41.8%) received XELOXIRI, 24 (35.8%) XELOX plus cetuximab and 15 (22.4%) FOLFIRI. In 58.2% of patients, four neoadjuvant cycles were administrated. Global median number of administered cycles was 4 [3-6] (Table 2). Eighteen patients (26.8%) reported some side effects to the chemotherapy, which did not contraindicate surgery. The most frequent complication was diarrhea, in six patients followed by neutropenia, in five patients. There was not any differences in complication rates between the three chemotherapy lines administered (P=0.65). After chemotherapy administration and before surgery, haemoglobin, leukocytes and platelets mean levels were 11.7×1012/L (8.6-15.3), 5.6×109/L (2.3-13.7) and 225×109/L [72-576] respectively. Median time between the start of chemotherapy and surgery was 107 days, and between the end of chemotherapy and surgery was 29 days. After neoadjuvant treatment, 53 patients (79.1%) achieved hepatic partial response, and 14 (20.9%) stability of the disease.
Full table
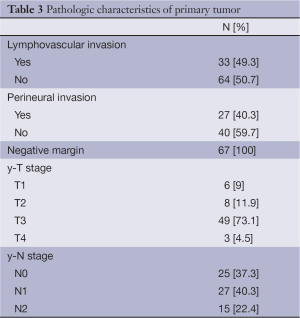
All patients underwent colon surgery and within those, eight patients (11.9%) underwent liver surgery simultaneously. Twenty-eight patients (41.8%), underwent liver surgery as a second procedure, and three patients (4.5%) underwent liver resection as the first procedure. Twenty-eight patients (41.8%) only underwent primary tumour surgery. In twelve cases (17.9%) a complementary surgery was conducted. Forty-nine sigmoidectomies/left-hemicolectomies, 14 right hemicolectomies, 2 transverse colon resections and 2 Hartmann operations were performed. In 23 patients (34.3%) a laparoscopic approach was elected. Pathologic characteristics of primary tumour are summarized in Table 3. Seven patients required blood transfusion during their hospital admission: one packed red blood cells (PRBC) on three patients and two PRBC on four patients. Median surgical time was 203 [75-469] minutes and median hospital admission was a total of 8 [3-29] days.
Full table
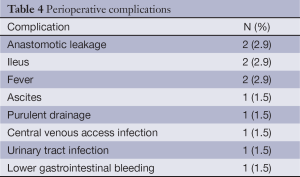
In Table 4 the complications reported in the first 30 days after surgery are shown. The complication rate was 16.4%, and no patient presented more than one complication. The morbidity predicted by POSSUM was 58.3%. There was not perioperative mortality, despite mortality prediction for P-POSSUM being 5.07%. No differences were observed between the chemotherapy regimen (P=0.72) or the kind of the surgery—simple or combined (P=0.58). After a median follow-up period of 25 months, the median OS was 31 (95% CI: 23-39) months. There were no cases of local relapse.
Full table
Discussion
This study aims to analyze the surgical morbidity secondaty to primary tumor resection after neoadjuvant chemotherapy, in a group of patients diagnosed with stage IV colon adenocarcinoma. The three most important chemotherapy regimens used in our hospital during the nine-year study-period were included: FOLFIRI, XELOXIRI, and XELOX together with cetuximab.
We included all complications that patients presented during chemotherapy treatment, hence global morbidity rate being 26.8%. It was not necessary to contraindicate surgery in any of the eighteen patients who presented complications. Blood levels of haemoglobin, leukocytes and platelets were analyzed and all of them were within the accepted range in order to perform a safe surgery.
Global complications that appeared after primary tumor resection were assessed, including cases where another surgical intervention was simultaneously performed. Five surgical and six clinical complications were observed. The complication global rate was 16.4%, which is comparable to another reported series (15-17) and much lower than the morbidity prediction for this group of patients (16.4% vs. 58.3%). Different speciality-specific POSSUM models have been developed, like O-POSSUM (18) or CR-POSSUM (19). POSSUM and P-POSSUM have been considered a good predictor of surgical complications (20) although some studies suggest an overprediction for colon cancer surgery with these scores (21).
No cases of surgical conversion from laparoscopic to open approach were observed and the reoperation rate was little lower than what has been described by other authors: 2.9% vs. 3.8-5.8% (15,22). These two cases of reoperation were secondary to an anastomotic leakage. One should mention the low blood transfusion requirement. The hospital admission period is within the same range as other colorectal surgery series. It is known that the previous comorbidity is a risk factor of surgical complications and hospital stay (23). Fifty five percent of our patients had some important copathology. No cases of perioperative mortality were registered.
If patients with unresectable metastatic colon cancer should undergo primary tumor resection still remains controversial. Some authors prefer primary chemotherapy (24-26), while others think that primary colectomy improves OS compared with only chemotherapy (27-29).
In stage IV CRC patients who complete all treatment steps, classical (primary tumour resection, liver metastases-directed chemotherapy followed by hepatic resection) and reversed (liver metastases-directed chemotherapy, hepatic resection and then, primary tumour resection) sequential managements have been associated with similar survival rates (30).
We can find some limitations in this study, such as the unicentric and retrospective character and the use of different chemotherapy regimens before surgery. On the other hand, this is a selected group of patients that were able to achieve the surgical treatment after chemotherapy.
Although chemotherapy treatment was administrated before surgery, our results are within the accepted postoperative complication limits of colorectal surgery. Used antithrombotic prophylaxis avoided cases of deep venous thrombosis or pulmonary thromboembolism, and respiratory physiotherapy avoided frequent pulmonary complications, such as pneumonia or respiratory failure in this study. We could confirm that chemotherapy does not indicate surgery contraindication nor increases postoperative morbi-mortality by a significant amount. This fact, showing the safety of preoperative chemotherapy, could be another reason to believe that neoadjuvant therapy could have a role to play in patients with locally advanced colon cancer.
Conclusions
Neoadjuvant chemotherapy as a systemic treatment for stage IV colon cancer does not associate with a high postoperative complication risk.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- van der Pool AE, Damhuis RA, Ijzermans JN, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis 2012;14:56-61. [PubMed]
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2008, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site, 2011. Accessed June 20, 2013.
- Golfinopoulos V, Salanti G, Pavlidis N, et al. Survival and disease-progression benefits with treatment regimens for advanced colorectal cancer: a meta-analysis. Lancet Oncol 2007;8:898-911. [PubMed]
- Adam R, de Haas RJ, Wicherts DA, et al. Concomitant extrahepatic disease in patients with colorectal liver metastases: when is there a place for surgery? Ann Surg 2011;253:349-59. [PubMed]
- Patel NA, Keenan RJ, Medich DS, et al. The presence of colorectal hepatic metastases does not preclude pulmonary metastasectomy. Am Surg 2003;69:1047-53; discussion 1053. [PubMed]
- Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol 2012;13:1152-60. [PubMed]
- Arredondo J, Pastor C, Baixauli J, et al. Preliminary outcome of a treatment strategy based on perioperative chemotherapy and surgery in patients with locally advanced colon cancer. Colorectal Dis 2013;15:552-7. [PubMed]
- Prytherch DR, Whiteley MS, Higgins B, et al. POSSUM and Portsmouth POSSUM for predicting mortality. Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity. Br J Surg 1998;85:1217-20. [PubMed]
- Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg 1991;78:355-60. [PubMed]
- Whiteley MS, Prytherch DR, Higgins B, et al. An evaluation of the POSSUM surgical scoring system. Br J Surg 1996;83:812-5. [PubMed]
- Maiello E, Gebbia V, Giuliani F, et al. FOLFIRI regimen in advanced colorectal cancer: the experience of the Gruppo Oncologico dell’Italia Meridionale (GOIM). Ann Oncol 2005;16 Suppl 4:iv56-60. [PubMed]
- Zarate R, Rodríguez J, Bandres E, et al. Oxaliplatin, irinotecan and capecitabine as first-line therapy in metastatic colorectal cancer (mCRC): a dose-finding study and pharmacogenomic analysis. Br J Cancer 2010;102:987-94. [PubMed]
- Moosmann N, Heinemann V.Cetuximab plus XELIRI or XELOX for first-line therapy of metastatic colorectal cancer. Clin Colorectal Cancer 2008;7:110-7. [PubMed]
- Cohen ME, Bilimoria KY, Ko CY, et al. Variability in length of stay after colorectal surgery: assessment of 182 hospitals in the national surgical quality improvement program. Ann Surg 2009;250:901-7. [PubMed]
- Hendren S, Birkmeyer JD, Yin H, et al. Surgical complications are associated with omission of chemotherapy for stage III colorectal cancer. Dis Colon Rectum 2010;53:1587-93. [PubMed]
- A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [PubMed]
- Tekkis PP, McCulloch P, Poloniecki JD, et al. Risk-adjusted prediction of operative mortality in oesophagogastric surgery with O-POSSUM. Br J Surg 2004;91:288-95. [PubMed]
- Tekkis PP, Prytherch DR, Kocher HM, et al. Development of a dedicated risk-adjustment scoring system for colorectal surgery (colorectal POSSUM). Br J Surg 2004;91:1174-82. [PubMed]
- Valenti V, Hernandez-Lizoain JL, Baixauli J, et al. Analysis of POSSUM score and postoperative morbidity in patients with rectal cancer undergoing surgery. Langenbecks Arch Surg 2009;394:55-63. [PubMed]
- Senagore AJ, Warmuth AJ, Delaney CP, et al. POSSUM, p-POSSUM, and Cr-POSSUM: implementation issues in a United States health care system for prediction of outcome for colon cancer resection. Dis Colon Rectum 2004;47:1435-41. [PubMed]
- Morris AM, Baldwin LM, Matthews B, et al. Reoperation as a quality indicator in colorectal surgery: a population-based analysis. Ann Surg 2007;245:73-9. [PubMed]
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8-27. [PubMed]
- Karoui M, Soprani A, Charachon A, et al. Primary chemotherapy with or without colonic stent for management of irresectable stage IV colorectal cancer. Eur J Surg Oncol 2010;36:58-64. [PubMed]
- Poultsides GA, Servais EL, Saltz LB, et al. Outcome of primary tumor in patients with synchronous stage IV colorectal cancer receiving combination chemotherapy without surgery as initial treatment. J Clin Oncol 2009;27:3379-84. [PubMed]
- Cirocchi R, Trastulli S, Abraha I, et al. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst Rev 2012;8:CD008997. [PubMed]
- Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg 2010;34:797-807. [PubMed]
- Karoui M, Roudot-Thoraval F, Mesli F, et al. Primary colectomy in patients with stage IV colon cancer and unresectable distant metastases improves overall survival: results of a multicentric study. Dis Colon Rectum 2011;54:930-8. [PubMed]
- Galizia G, Lieto E, Orditura M, et al. First-line chemotherapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg 2008;143:352-8; discussion 358. [PubMed]
- Andres A, Toso C, Adam R, et al. A survival analysis of the liver-first reversed management of advanced simultaneous colorectal liver metastases: a LiverMetSurvey-based study. Ann Surg 2012;256:772-8; discussion 778-9. [PubMed]


