Multiple primary malignancies in patients with anal squamous cell carcinoma
Introduction
Improvements in early intervention and treatment modalities have led to a 26% decline in cancer death rates in the United States (US) in the past two decades (1). This has resulted in about 15 million cancer survivors alive in the US in 2016, a number expected to rise to 20 million by 2026 (2). However, cancer survivors are predisposed to develop new cancers due to risk factors such as genetic disorders and somatic mutations resulting from prior chemotherapy or radiotherapy (3,4). Cancer survivors are now a significant subset of patients presenting with new cancers, accounting for 17% of new cancer cases in the US. An analysis of 10 high-incidence cancer sites using the Surveillance, Epidemiology, and End Results (SEER) registry showed that 55% of patients with two cancers died of their second primary malignancy (SPM), a greater percentage than patients with only a single primary who died of that cancer (5).
Patients with squamous cell carcinoma of the anus (SCCA) are at risk of SPM development due to favorable long-term survival, high association with human papillomavirus (HPV) (6), and receipt of chemoradiotherapy. A recent study found the 5-year age-standardized relative survival of SCCA patients to be 65.9%. Furthermore, half of SCCA patients present with localized disease which portends a 5-year relative survival rate of approximately 80% (7). After an index SCCA, the rate of developing an HPV-related SPM is significantly increased (8). A study from France found an increase in global SPM in men but not women after index SCCA, hypothesized to be due to the higher prevalence of human immunodeficiency virus (HIV) in men (9).
Prior studies examining the risk of SPM after an index SCCA have used large datasets such as the SEER registry and excluded survivors of previous primaries and developers of synchronous primaries. By contrast, we conducted a single-institution study to provide a more complete representation of survivorship status, survival trends, and multiple cancer risk in SCCA patients.
Materials and methods
Study participants
An IRB-approved retrospective review identified 46 patients with SCCA treated with curative intent at Robert Wood Johnson University Hospital (RWJUH) and Rutgers Cancer Institute of New Jersey from January 2006 to June 2017. Additional malignancies were recorded through chart review and the RWJUH tumor registry. Multiple malignancies in a single patient were biopsy-proven to be morphologically and histologically different. Per National Comprehensive Cancer Network guidelines, patients were tested for HIV at time of SCCA diagnosis.
Patients received definitive chemoradiotherapy, with the exception of one patient treated with curative local excision. Standard of care consisted of 2 cycles of mitomycin-C (10–15 mg/m2 on days 1 and 29) and 5-fluorouracil (5-FU) (1,000 mg/m2 continuous infusion on days 1–4 and 29–32) with radiotherapy to 50.4–54 Gy to the pelvic lymph nodes and primary tumor. T3–T4 tumors received a boost to 60 Gy. For five patients, daily capecitabine was substituted for infusional 5-FU (10).
Definitions
We defined an “index primary malignancy” (IPM) as the first diagnosed cancer. A “second primary malignancy” (SPM) was defined as a biopsy-confirmed biologically distinct primary cancer that developed after IPM diagnosis. An SPM is “synchronous” if diagnosed <6 months after IPM diagnosis and “metachronous” if diagnosed ≥6 months after IPM diagnosis.
Data analysis
Patients were classified into one of three groups: prior IPM, SCCA-only and SPM after an index SCCA. Summary statistics of baseline characteristics and details of additional malignancies were provided for each group. Univariate logistic regression was used to assess associations between baseline characteristics [gender, age, race (white vs. non-white), stage (III/IV vs. others), smoking status (ever smoked vs. never smoked)] and either presence of ≥2 malignancies among all patients or development of SPM among index SCCA patients. Overall survival was estimated with the Kaplan-Meier method, measured from end of radiotherapy to date of death or last known follow-up. P<0.05 was considered statistically significant. Analyses were performed using SAS version 9.4 (Cary, NC, USA).
The datasets generated and/or analysed during the current study are available from the corresponding author upon reasonable request.
Results
Of the 46 patients treated definitively for SCCA, a total of 18 (39.2%) patients had multiple primary malignancies. Nine (19.6%) patients had a prior IPM, 9 (19.6%) developed SPMs after an index SCCA, and 28 (60.9%) had SCCA only. Six patients had ≥3 total malignancies. No baseline characteristics (Table 1) correlated with presence of ≥2 malignancies (among all patients) or development of SPM (among index SCCA patients).
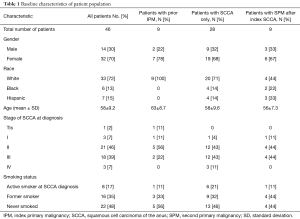
Full table
Prior IPM patients
Among the 9 patients with a prior IPM, 7 were female. The median latency from IPM to SCCA diagnosis was 141.6 months (range, 20.5–296.7 months). All patients had a latency period of >120 months except for two who developed SCCA after 20 and 30 months. Of note, these two patients also developed third cancers within three months of SCCA diagnosis.
The most common IPMs were breast (n=4) and gynecologic cancers [endometrial (n=2) and vulvar (n=1)] (Table 2). Malignancies in the pelvic radiation field (gynecological and rectal) did not receive radiation, so subsequent development of SCCA was not clearly related to radiotherapy.
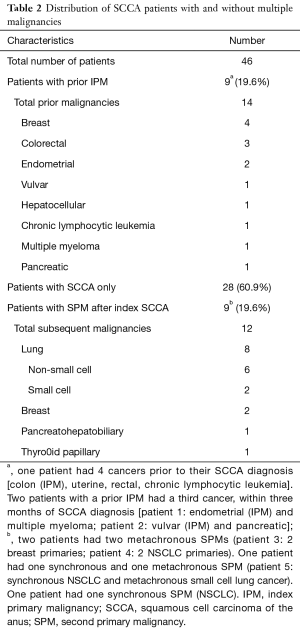
Full table
Index SCCA patients
Of the 37 total patients with an index SCCA, 9 (23.7%) patients had ≥1 SPM (Table 2). The median latency from an index SCCA to first SPM diagnosis was 36.3 months (range, 1–146.1 months). Two patients had synchronous SPMs. None of the SPMs were found to be in the pelvic radiation field or HPV-related.
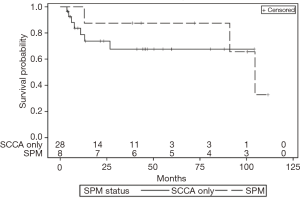
Median follow-up for SCCA-only patients and SPM patients was 45 and 102 months, respectively. Of the 28 SCCA-only patients, 6 died of SCCA within 10 months of treatment (Figure 1). Of the 9 SPM patients, 4 died from their SPM 12–36 months after diagnosis of their SPM. Three of these deaths were from stage IV lung cancer and one was from stage IV pancreatobiliary adenocarcinoma. There were not enough death events to correlate SPM status to survival.
Of the 9 patients who developed SPMs, 6 (67%) developed lung cancers. Three of these patients had no smoking history, of which 2 developed multiple lung primaries. Non-small cell lung cancer (NSCLC) (n=6) and SCLC (n=2) accounted for 46% and 15% of the total number of SPMs, respectively. Four lung primaries were diagnosed at or progressed to stage IV.
Four patients were HIV positive with undetectable viral load while on antiretroviral medication. Of these four patients, one developed SCLC and NSCLC, and one developed 2 primary breast cancers.
Discussion
We discovered an elevated rate of multiple primary malignancies in SCCA patients. Of 46 total patients, 9 (19.6%) had prior IPMs and 9 (19.6%) developed SPMs after an index SCCA. The study is limited by lack of genomic analysis data and small sample size. However, several risk factors may play a role including HPV infection, HPV-related or treatment-related immunosuppression, somatic mutations due to chemotherapy, and genetic factors.
HPV-related and treatment-related general immunosuppression may predispose patients to second cancers in our IPM and SPM cohorts. HPV16-specific T-cell frequency lowers dramatically after radical/curative treatment in oropharyngeal cancer patients (11). While information on HPV status was limited, HPV prevalence in anal cancer populations can be as high as 90% (6). Among patients with a prior IPM, we hypothesize that treatment-induced immunosuppression of previous cancers could lead to HPV persistence after infection, thus increasing risk of SCCA development (12). Though HPV infection is a risk factor for developing HPV-related SPMs after an index SCCA (8), there were none in our SPM patient cohort. However, virally-induced immunosuppression can indirectly promote tumor development (13); combined with treatment-induced immunosuppression, the risk for multiple cancers may increase.
The most common SPM was lung cancer, aligning with a SEER analysis which found increased risk of lung cancer after an index SCCA (14). One study suggests lung cancer is the most common SPM in survivors of common cancers, and suggested risk factors include exposure to etiological agents such as tobacco smoke and chemotherapy (5). However, 3 of the 6 patients who developed lung cancer never smoked, and 2 of the never-smokers developed multiple lung SPMs. Risk factors remain uncertain, but immunosuppression, somatic mutations from chemotherapy, or genetic factors may play a role.
SCCA-only patients were more likely to die soon after treatment from SCCA progression, whereas SPM patients tended to die soon after their SPM diagnosis. While survival of the SCCA-only group stabilizes after about one year, survival of the SPM group worsens (Figure 1). SPM patients were cured of SCCA, but their SPMs generally had a worse baseline prognosis.
In conclusion, SCCA patients are often either survivors of previous cancers or develop later malignancies. As SCCA outcomes continue to improve and incidence rises (1,15), patients will be at increased risk for developing SPMs, an area which has not garnered sufficient study. We believe our findings provide a basis for examining these trends in a larger cohort. There is a possible genetic link between SCCA and lung cancer SPMs that should be investigated further. Identifying factors predisposing SCCA patients to SPMs may aid in the development of screening or surveillance tools, particularly for patients who respond to initial treatment.
Acknowledgements
Funding: The work was supported by the National Cancer Institute Cancer Center Support Grant (P30CA072720-15).
Footnote
Conflicts of Interest: Salma K. Jabbour has received research grants from Merck and Nestle. The other authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was conducted at the Rutgers Cancer Institute of New Jersey and approved by the Rutgers Institutional Review Board (Pro20170001775). Informed consent was waived, as this was a retrospective study.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Cancer Treatment & Survivorship Facts & Figures: 2016-2017. Atlanta, GA: American Cancer Society, 2016.
- Berrington de Gonzalez A, Curtis RE, Kry SF, et al. Proportion of second cancers attributable to radiotherapy treatment in adults: a cohort study in the US SEER cancer registries. Lancet Oncol 2011;12:353-60. [Crossref] [PubMed]
- Morton LM, Onel K, Curtis RE, et al. The rising incidence of second cancers: patterns of occurrence and identification of risk factors for children and adults. Am Soc Clin Oncol Educ Book 2014.e57-67. [Crossref] [PubMed]
- Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer 2016;122:3075-86. [Crossref] [PubMed]
- Saraiya M, Unger ER, Thompson TD, et al. US assessment of HPV types in cancers: implications for current and 9-valent HPV vaccines. J Natl Cancer Inst 2015;107. [Crossref] [PubMed]
- Razzaghi H, Saraiya M, Thompson TD, et al. Five-year relative survival for human papillomavirus-associated cancer sites. Cancer 2018;124:203-11. [Crossref] [PubMed]
- Nelson RA, Lai LL. Elevated risk of human papillomavirus-related second cancers in survivors of anal canal cancer. Cancer 2017;123:4013-21. [Crossref] [PubMed]
- Neumann F, Jegu J, Mougin C, et al. Risk of second primary cancer after a first potentially-human papillomavirus-related cancer: A population-based study. Prev Med 2016;90:52-8. [Crossref] [PubMed]
- Goodman KA, Julie D, Cercek A, et al. Capecitabine With Mitomycin Reduces Acute Hematologic Toxicity and Treatment Delays in Patients Undergoing Definitive Chemoradiation Using Intensity Modulated Radiation Therapy for Anal Cancer. Int J Radiat Oncol Biol Phys 2017;98:1087-95. [Crossref] [PubMed]
- Al-Taei S, Banner R, Powell N, et al. Decreased HPV-specific T cell responses and accumulation of immunosuppressive influences in oropharyngeal cancer patients following radical therapy. Cancer Immunol Immunother 2013;62:1821-30. [Crossref] [PubMed]
- Torres-Poveda K, Bahena-Roman M, Madrid-Gonzalez C, et al. Role of IL-10 and TGF-beta1 in local immunosuppression in HPV-associated cervical neoplasia. World J Clin Oncol 2014;5:753-63. [Crossref] [PubMed]
- Morales-Sanchez A, Fuentes-Panana EM. Human viruses and cancer. Viruses 2014;6:4047-79. [Crossref] [PubMed]
- Shah BK, Budhathoki N. Second Primary Malignancy in Anal Carcinoma--A US Population-based Study. Anticancer Res 2015;35:4131-4. [PubMed]
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]


