Reclassification of lesions in biopsies by fine-needle aspiration of pancreas and biliary tree using Papanicolaou classification
Introduction
Pancreatic cancer ranks 12th among malignant neoplasms in the United States (1) and is the fourth leading cause of cancer-related deaths (2,3). Major symptoms include obstructive jaundice, abdominal pain, weight loss, acute pancreatitis, diabetes of recent onset and long-term deterioration (4-6).
In patients with suspected cancer, it is recommended to perform a three-phase pancreatic tomography and endoscopic ultrasound (EUS) in order to stratify and evaluate the possibility of primary surgical resection, an advantage of EUS is that fine-needle aspiration (FNA) can be performed as an integral part of the examination, allowing the acquisition of tissue and a prompt diagnosis (7-11).
Currently, the FNA obtained by EUS (FNA-EUS) is an excellent diagnostic tool to obtain cytopathological evidence of pancreatic and bile duct tumors, contributing to considerable improvements in the management of patients with pancreatic and biliary neoplasms (12-14). This method has a very high specificity (71–100%) with 0–5% false-positives; however, the false-negative interpretation ranges between 4–14% as a result of inadequate sampling, errors of interpretation and the presence of technical factors. That affects the sensitivity of the test, creating the need to implement a unified system of terminology (8,14-16). The Papanicolaou Cytopathology Society proposed a new classification for the report of cytology of pancreas and bile duct, dividing the lesions in six categories (16-21) and using auxiliary studies for the cytological diagnosis that includes biochemical, molecular, imaging studies, special stains of histochemistry and immunocytochemistry for an integral and personalized diagnosis (21-28).
One advantage of this new classification is the standardization of the terms between the different medical centers and the management guidelines for each category, facilitating communication between the interdisciplinary medical team (21).
Our objective was to determine the diagnostic performance of the FNA-EUS when applying the classification of the Papanicolaou Cytology Society for pancreas and bile duct cytologies.
Methods
Ethic statement
This work was authorized by the ethics and research committee of our institution, with a waiver of informed consent, because of its retrospective nature (approval number: Rev/17/79). Likewise, the anonymity of the participants is guaranteed.
Population
A retrospective cross-sectional study was conducted, which included all the consecutively biopsies by aspiration with FNA-EUS of pancreas and bile duct in the National Cancer Institute of Mexico in 2016. Cases with at least 6 months of follow-up and with histological report were included. Biopsies by aspiration of liver, peripancreatic and digestive tube nodes were excluded.
Obtaining and preparing the simple
The FNA-EUS procedure is routinely performed by an experienced endoscopist with a linear echoendoscope (Olympus Lineal 180) with a thin 22 G needle, with an average of 2–3 passes per session. The aspirate is immediately distributed in glass slides for later staining with Hemacolor and Papanicolaou techniques.
Cytologic evaluation and processing of the cell-block
The first cytological interpretation to decide the sufficiency of the sample was made by a cytopathologist in training. The final interpretation was performed independently by three cytopathologists without knowing the clinical data and histopathologic results. The original cytological diagnoses were reclassified based on the six categories proposed by the Papanicolaou Society: (I) non-diagnostic, (II) benign lesion, (III) atypical, (IV) benign neoplasia and other neoplasms, (V) suspicious of malignancy, and (VI) malignant neoplasm. To classify mucinous cystic lesions, a cut-off level of ACE was considered to be 192 ng/dL. The concordance with the final histological diagnosis was determined in all cases where resection samples were available. The discrepant cases were classified as partial or no agreement and subsequently they were reviewed to evaluate the reasons for discrepancy.
Statistical analysis
The statistical analysis was performed in SPSS ver. 23 (IBM, Armonk, NY, USA). The continuous variables were expressed as medians and interquartile range, and compared between groups using the Mann-Whitney test. The qualitative variables were summarized by counting and percentages, and the groups compared by Fisher’s exact test. For both tests a value of P<0.05 was considered significant. The diagnostic performance (sensitivity, specificity, positive predictive value, negative predictive value) and cytohistological correlation of the new classification were determined using the histopathological study as a reference standard. The atypical, suspicious malignancy and malignancy results were considered as a positive cytological diagnosis.
Results
Demographic and clinicopathological characteristics of patients
We identified 134 cases of FNA-EUS with a median age of 59 years (IQR, 46–65 years), 88 were women (66%) and 46 men (34%). Regarding EUS characteristics, 83% of cases were solid lesions and 17% cystic lesions, of which 114 cases consisted of purely solid lesions, 2 cystic lesions and 15 mixed lesions. In 93 (42%) cases the most frequent symptom was pain, 70 (31%) cases had jaundice and 59 (27%) cases weight loss. The presence of pain and/or jaundice occurred in all diagnostic categories, however, weight loss was not found in those classified in categories 2 and 4.
Cytology analysis
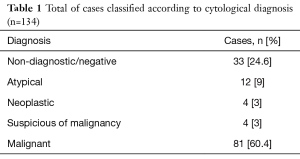
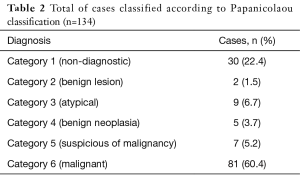
The cytological diagnoses issued before the application of the new classification included: 33 (24.6%) non-diagnostic and negative samples, 12 (9%) atypical lesions, 4 (3%) neoplasms, 4 (3%) suspicious of malignancy and 81 (60.4%) malignant (Table 1). Applying the new classification proposed by the Papanicolaou Cytopathology Society, 30 cases (22.4%) were reclassified in category 1, 2 cases (1.5%) category 2, 9 cases (6.7%) category 3, 5 cases (3.7%) category 4, 7 cases (5.2%) category 5, and finally 81 cases (60.4%) category 6 (Table 2). Of 18 cases that initially presented a descriptive result, 8 (44.4%) cases were reclassified to category 1 (non-diagnostic) and of the remaining 10 (55.6%) that previously described cells with atypia, 7 cases were included in category 3 (atypia) and 3 cases were reclassified in category 5 (suspected of malignancy).
Full table
Full table
Considering the previous nomenclature, of the 33 cases negative/non-diagnostic, 30 were reclassified as category 1, since they presented only glandular epithelium without alterations, reactive cells, hemorrhagic background and/or artifices; of these cases, 12 had histopathological study: 7 cases with adenocarcinomas, 2 cases with microcystic serous cystadenoma, 1 case with neuroendocrine carcinoma, 1 case with pseudopapillary solid neoplasia, 1 case with nonspecific inflammation. Two cases were classified as category 2, one of them with cytological characteristics compatible with a pancreatic pseudocyst, with a histopathological report of a pancreatic pseudocyst, and a case in which mild chronic inflammation and fibrosis were observed cytologically, with histopathological report also inflammatory. Finally, one case based on the cytological, EUS and biochemical characteristics [with serologic carcinoembryonic antigen (CEA) levels >192 ng/mL], was reclassified to category 4.
There were 12 cases previously interpreted as atypical, which according to the new guidelines, 9 cases remained category 3, of which 4 have a histopathological report: 1 adenocarcinoma, 1 carcinoma with foci of mucinous differentiation, 2 neuroendocrine neoplasms grade 2, and the remaining three cases were reclassified to category 5. Of the four cases that were diagnosed before the reclassification as neuroendocrine neoplasia or solid pseudopapillary tumor, they were reclassified in category 4. Two cases with a cytological diagnosis of neuroendocrine neoplasia have a histopathological report of only one: neuroendocrine neoplasia grade 1. Two cases cytologically with a diagnosis of pseudopapillary solid neoplasm, both with the same histopathological report. Finally, a case of the previous negative/non-diagnosis nomenclature was added, which had CEA >192 ng/mL, consisted of a predominantly cystic lesion with focal solid areas and cytologically represented by reactive epithelium, with a Histopathological report of mucinous papillary intraductal neoplasia.
For the category suspicious of malignancy, the 4 cases previously diagnosed were maintained in this category and 3 were added with the reclassification, there is only one histopathological report of adenocarcinoma. All the positive interpretations for malignancy remained as malignant in category 6, counting on the histopathological report of 12 cases: 7 adenocarcinomas, 3 cholangiocarcinoma, 2 metastases (1 mixed cystadenocarcinoma with papillary and high-grade clear cell areas and 1 cell carcinoma of Merkel).
Regarding the surgical follow-up of all the patients, there was a histopathological report of 35 cases, 13 (37%) product of pancreatoduodenectomy and 22 biopsy samples (63%).
From the cases with histologic tissue available, 21 (91.3%) cases had cytohistologic concordance and 2 (8.7%) discordance. The reasons for discrepancy were due to sampling error, one of them with scarce atypical material, the other case with partial agreement because cytological atypical cylindrical epithelium was observed and in histology a grade 2 neuroendocrine neoplasia was identified with infiltration in the body and tail, and low grade mucinous intraepithelial neoplasia.
In general, sensitivity and specificity were 100% and 75% respectively, positive predictive value 88%, and negative predictive value 100%. The 12 cases that were classified cytologically as category 1, were due to sampling error, since the stretches contained normal and reactive glandular epithelium, and with artifices, this material was not representative of the lesion presented in the clinical/EUS context.
Discussion
The purpose of this new classification is to standardize the cytological report of pancreatic and biliary tract lesions, facilitating communication between the interdisciplinary medical team. The sensitivity and specificity of the FNA-EUS in the literature is variable, depending on many factors such as the sample size, nature of the lesions (cystic vs. solid) and the definition of “positive” in the statistical analysis (29-31).
Hewitt et al. (32), in a meta-analysis that included 33 studies with a total of 4,984 patients, evaluated the diagnostic accuracy of FNA-EUS to detect pancreatic cancer. The sensitivity for malignant cytology was 85% and specificity 98%. Including as positive the results of atypical cytology and suspicious of malignancy, the sensitivity increased to 91%, however, the specificity was reduced to 94%.
Bergeron, in a retrospective study that included 1,212 cases of FNA-EUS of both cystic and solid pancreatic lesions, reported a sensitivity of 83.2%, specificity of 85.9%, positive predictive value of 95.5%, and negative predictive value of 56.1% (29).
Eloubeidi et al. evaluated 101 cases of FNA-EUS of patients with pancreatic solid tumors, with a sensitivity of 94.7%, specificity 100%, positive predictive value of 100% and negative predictive value of 85.2% (31). In the present study, a high sensitivity of 100% was obtained, however, specificity of 75%, with a positive predictive value of 88% and a negative predictive value of 100%. False-positive cases of pancreatic FNA-EUS are rare and generally represent <1% of all malignant cases, consisting mainly of cells with atypia, and atypical mucinous cell groups (29,30). In our study, two false cases were presented positive, due to sampling errors.
A fundamental premise to implement a wide use of the FNA-EUS is not to lose a “positive” case. However, false-negative cases are common, coinciding with different series such as Bergeron et al. reporting a false negative rate of 43.3%, defining it as cytologically negative cases in which neoplastic histology was found (29). Woolf et al. reported similar cases, with 19 of 39 cases (48.7%) cytologically negative with a histological diagnosis of neoplasia (30). Saieg et al., in 6 cytologically negative cases with positive histology for neoplasia, 5 were considered sampling errors and 1 with error of interpretation (14). In the present study, from the 12 cases in the category “non-diagnostic”, one case was due to sampling error.
In our study, a greater category change was observed in the “descriptive”, “non-diagnostic” and “atypical” diagnoses, and with minimal change in the “suspicious” and “malignant categories”. Saieg et al. reclassified 155 studies according to the New Classification of the Papanicolaou Society of Cytopathology, all malignant cytologies (61 cases, 39%) remained within the same category of malignancy (14).
Comparing the two terminologies, the new nomenclature reclassified in a clear and simple way the “non-diagnostic” lesions in category 1 and the “benign lesions” in category 2, which includes specific pathologies that can be diagnosed cytologically as benign (16,21). For example, the pancreatic pseudocyst, with one case in our series.
Another aspect to be highlighted includes the cystic lesions, in the new proposal it is mentioned that the absence of epithelial cells does not necessarily mean that a sample is not diagnostic, such as the pancreatic pseudocyst. It is also the case of lesions previously interpreted as non-diagnostic/negative which, now considering the cystic context of the lesion, with high CEA levels, should be interpreted as category 4 (14,16,31). In our study, one case was reclassified from “non-diagnostic” to category 4, with a histopathological report of intraductal papillary mucinous neoplasia. Bergeron et al. affirm in their study that the “triple test” (association of clinical, radiological and cytological findings) is currently the best method to classify mucinous lesions and we agree (29).
It is important to point out that, as in the literature, the greatest relevance was presented in the categories that are usually described as a gray area, since in these cases a definitive diagnosis of malignancy cannot be interpreted. According to our former classification, most of the borderline cases were considered as atypical lesions (12 cases). With the reclassification, a quarter of them were considered category 5, considerably increasing the risk of malignancy from 58% to 84% (16). In the literature, the cases with cytological diagnosis of atypia constitute from 1% to 17%, being the majority >10% (29). In our series, the 9 cases classified category 3 represented the 6.71%.
In addition to the re-evaluation of the sample, repeating EUS-guided sampling is another option that has been suggested for pancreatic lesions with inconclusive results. Additionally, two retrospective studies found that, for indeterminate cytopathological diagnoses, clinical conditions such as weight loss and obstructive symptoms were associated with a diagnosis of malignancy as it was in our study (33).
Conclusions
We confirmed that FNA-EUS interpreted according to the Papanicolaou Cytopathology Society Classification, is an accurate method to evaluate pancreatic and biliary tract lesions, with a high positive predictive value. The correlation between the EUS, biochemical data, and cytological findings is essential to classify neoplasms, especially those with mucinous characteristics. It is important to emphasize that the cytopathological study in the diagnosis of pancreatic and bile duct lesions can significantly influence the clinical management of these patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: This study was approved by the Ethics in Investigation Board of Mexico’s National Cancer Institute with a waiver of informed consent (approval No. 17/79).
References
- Singh A, Faulx AL. Endoscopic Evaluation in the Workup of Pancreatic Cancer. Surg Clin N Am 2016;96:1257-70. [Crossref] [PubMed]
- SEER Stat Fact Sheets: Pancreatic Cancer. NCI Surveillance, Epidemiology, and End Results Program 2005-2011. Bethesda (MD): National Cancer Institute. Available online: http://seer.cancer.gov/statfacts/html/pancreas.html
- Kalogeraki A, Papadakis GZ, Tamiolakis D, et al. EUS Fine Needle Aspiration Biopsy in the Diagnosis of Pancreatic Adenocarcinoma. Rom J Intern Med 2016;54:24-30. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma. Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1028-61. [Crossref] [PubMed]
- Cooperman AM, Iskandar ME, Wayne MG, et al. Prevention and Early Detection of Pancreatic Cancer. Surg Clin N Am 2018;98:1-12. [Crossref] [PubMed]
- Adler D, Max Schmidt C, Al-Haddad M, et al. Clinical Evaluation, Imaging Studies, Indications for Cytologic Study, and Preprocedural Requirements for Duct Brushing Studies and Pancreatic FNA: The Papanicolaou Society of Cytopathology Recommendations for Pancreatic and Biliary Cytology. Diagn Cytopathol 2014;42:325-32. [Crossref] [PubMed]
- Pitman MB, Layfield LJ. Guidelines for Pancreaticobiliary Cytology from the Papanicolaou Society of Cytopathology: A Review. Cancer Cytopathol 2014;122:399-411. [Crossref] [PubMed]
- Peláez LM, Blancas JM, Barrera-Torres R, et al. Papel actual de la endoscopia en patología pancreática. Endoscopia 2013;25:56-65.
- Feldman MK, Gandhi NS. Imaging Evaluation of Pancreatic Cancer. Surg Clin N Am 2016;96:1235-56. [Crossref] [PubMed]
- Burk KS, Lo GC, Gee M, et al. Imaging and Screening of Pancreatic Cancer. Radiol Clin N Am 2017;55:1223-34. [Crossref] [PubMed]
- Arango LA, Diaz CP. Endoscopic ultrasound in pancreatic disease. Rev Med Clin Condes 2015;26:634-48.
- Brugge W, Dewitt J, Klapman JB, et al. Techniques for Cytologic Sampling of Pancreatic and Bile Duct Lesions. Diagn Cytopathol 2014;42:333-7. [Crossref] [PubMed]
- Matsubayashi H, Matsui T, Yabuuchi Y, et al. Endoscopic ultrasonography guided-fine needle aspiration for the diagnosis of solid pancreaticobiliary lesions: Clinical aspects to improve the diagnosis. World J Gastroenterol 2016;22:628-40. [Crossref] [PubMed]
- Saieg MA, Munson V, Colletti S, et al. The Impact of the New Proposed Papanicolaou Society of Cytopathology Terminology for Pancreaticobiliary Cytology in Endoscopic US-FNA: A Single Institutional Experience. Cancer Cytopathol 2015;123:488-94. [Crossref] [PubMed]
- Perez-Machado MA. Pancreatic cytology: standardized terminology and nomenclature. Cytopathology 2016;27:157-60. [Crossref] [PubMed]
- Pitman M, Lester J. The Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology. Switzerland: Springer International, 2005.
- Pitman MB, Centeno BA, Ali SZ, et al. Standardized terminology and nomenclature for pancreatobiliary cytology: the Papanicolaou Society of Cytopathology guidelines. Diagn Cytopathol 2014;42:338-50. [Crossref] [PubMed]
- Kurtycz D, Tabatabai ZL, Michaels C, et al. Postbrushing and fine-needle aspiration biopsy follow-up and treatment options for patients with pancreatobiliary lesions: the Papanicolaou Society of Cytopathology guidelines. Diagn Cytopathol 2014;42:363-71. [Crossref] [PubMed]
- Collins JA, Ali SZ, VandenBussche CJ. Pancreatic Cytopathology. Surg Pathol Clin 2016;9:661-76. [Crossref] [PubMed]
- McKinley M, Newman M. Observations on the application of the Papanicolaou Society of Cytopathology standardized terminology and nomenclature for pancreaticobiliary cytology. Pathology 2016;48:353-6. [Crossref] [PubMed]
- Bibbo M. Comprehensive Cytopathology. 4 ed. Philadelphia: Elsevier, 2015.
- Layfield LJ, Ehya H, Filie AC, et al. Utilization of ancillary studies in the cytologic diagnosis of biliary and pancreatic lesions: the Papanicolaou Society of Cytopathology guidelines for pancreatobiliary cytology. Diagn Cytopathol 2014;42:351-62. [Crossref] [PubMed]
- Olson MT, Ali SZ. Cytotechnologist on-site evaluation of pancreas fine needle aspiration adequacy: comparison with cytopathologists and correlation with the final interpretation. Acta Cytol 2012;56:340-6. [Crossref] [PubMed]
- Díaz Del Arco C, Esteban López-Jamar JM. Fine-needle aspiration biopsy of pancreatic neuroendocrine tumors: Correlation between Ki-67 index in cytological samples and clinical behavior. Diagn Cytopathol 2017;45:29-35. [Crossref] [PubMed]
- Sigel C, Reidy-Lagunes D, Lin O, et al. Cytological features contributing to the misclassification of pancreatic neuroendocrine tumors. J Am Soc Cytopathol 2016;5:266-76. [Crossref]
- Smith AL, Abdul-Karim FW, Goyal A. Cytologic Categorization of Pancreatic Neoplastic Mucinous Cysts With an Assessment of the Risk of Malignancy: A Retrospective Study Based on the Papanicolaou Society of Cytopathology Guidelines. Cancer Cytopathol 2016;124:285-93. [Crossref] [PubMed]
- Ajaj Saieg M, Munson V, Colletti S, et al. Impact of Pancreatic Cyst Fluid CEA Levels on the Classification of Pancreatic Cysts Using the Papanicolaou Society of Cytology Terminology System for Pancreaticobiliary Cytology. Diagn Cytopathol 2017;45:101-6. [Crossref] [PubMed]
- Chhieng D. Pancreatic Cytopathology. Switzerland: Springer, 2007.
- Bergeron JP, Perry KD, Houser PM, et al. Endoscopic ultrasound-guided pancreatic fine-needle aspiration: Potential pitfalls in one institution's experience of 1212 procedures. Cancer Cytopathol 2015;123:98-107. [Crossref] [PubMed]
- Woolf KM, Liang H, Sletten ZJ, et al. False-negative rate of endoscopic ultrasound-guided fine-needle aspiration for pancreatic solid and cystic lesions with matched surgical resections as the gold standard: one institution’s experience. Cancer Cytopathol 2013;121:449-58. [Crossref] [PubMed]
- Eloubeidi MA, Jhala D, Chhieng DC, et al. Yield of endoscopic ultrasound guided fine needle aspiration biopsy in patients with suspected pancreatic carcinoma. Cancer 2003;99:285-92. [Crossref] [PubMed]
- Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 2012;75:319-31. [Crossref] [PubMed]
- Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline – Updated January 2017. Endoscopy 2017;49:695-714. [Crossref] [PubMed]


