Retrospective analysis of efficacy and safety of Gemcitabine-based chemotherapy in patients with metastatic pancreatic adenocarcinoma experiencing disease progression on FOLFIRINOX
Introduction
Advanced pancreatic adenocarcinoma still carries a dismal prognosis; less than 1% of patients are alive 5 years after the discovery of metastasis (1). Despite recent advances in cytotoxic chemotherapy in the first-line setting (2,3), virtually all patients experience disease progression when treated with FOLFIRINOX or Gemcitabine plus Nab-Paclitaxel. Hence, patients frequently need additional treatments to palliate symptoms and extend survival.
After single-agent Gemcitabine, only one randomized trial has properly compared survival outcomes of patients undergoing second-line treatment with those of patients not actively treated (4). In the CONKO-003, treatment with OFF (Oxaliplatin, 5-fluorouracil and folinic acid) was associated with increased overall survival (OS). Three additional randomized controlled trials have evaluated the role of polychemotherapy after Gemcitabine monotherapy, and two of them favored 5-fluorouracil-based combinations over single-agent 5-fluorouracil in this scenario (5-7). Thus, there is a shortage of prospective studies assessing the role of second-line treatment, especially that of Gemcitabine-based chemotherapy after disease progression on FOLFIRINOX (8).
Likewise, most data regarding the use of Gemcitabine-based chemotherapy after FOLFIRINOX stem from retrospective studies. They report response rates (RRs) ranging from 10% to 30% and median OS times ranging from 3.6 to 12.4 months (8-16). In these studies, patients treated with Gemcitabine plus Nab-Paclitaxel seemed to fare better than those treated with Gemcitabine monotherapy. However, such studies were performed in rather heterogeneous populations, including patients treated with FOLFIRINOX for locally advanced disease, and individuals who had FOLFIRINOX interrupted due to toxicity concerns—not experiencing disease progression. Consequently, further studies are needed to evaluate the potential benefits and harms of Gemcitabine-based chemotherapy in this setting.
Herein we report the results of a retrospective study including 42 patients with metastatic pancreatic adenocarcinoma (MPA) treated with Gemcitabine-based chemotherapy following disease progression on FOLFIRINOX. We aimed to assess the efficacy and the toxicity profile of Gemcitabine-based chemotherapy in this setting.
Methods
Design
This is a retrospective study carried out in a single cancer-specialized hospital in Brazil. It was based on routinely collected data retrieved from the electronic charts of patients with MPA submitted to Gemcitabine-based chemotherapy following disease progression on FOLFIRINOX. Data were collected from August 2017 to January 2018. This study was approved by the A.C. Camargo Cancer Center Internal Ethics Review Board (CAAE 88206718.7.0000.5432).
Patients
We included patients with the following characteristics: age ≥18 years, with pathologically confirmed diagnosis of MPA from January 1st 2010 to December 31th 2016 and disease progression on first-line FOLFIRINOX according to RECIST 1.1, Eastern Cooperative Oncology Group (ECOG) performance status 0–2, and treatment with at least one cycle of Gemcitabine-based chemotherapy in second or further lines of chemotherapy. Patients who underwent treatment outside A.C. Camargo Cancer Center were excluded.
Treatment
Patients were treated with FOLFIRINOX in the first-line setting, with either standard or attenuated doses. In further lines of treatment, Gemcitabine was used as single-agent or in combination. We considered patients as been treated with single-agent Gemcitabine when no other cytotoxic agent was used along with it at any time. Gemcitabine-based combination regimens included Gemcitabine plus Nab-Paclitaxel, Gemcitabine plus Cisplatin and GEMOX (gemcitabine plus Oxaliplatin). Gemcitabine monotherapy consisted of Gemcitabine IV 1,000 mg/m2 on days 1, 8 and 15 of a 28-day cycle. Gemcitabine plus Nab-Paclitaxel consisted of Gemcitabine IV 1,000 mg/m2 and Nab-Paclitaxel IV 125 mg/m2 on days 1, 8 and 15 of a 28-day cycle. Gemcitabine + Cisplatin consisted of Gemcitabine 1,000 mg/m2 IV and Cisplatin IV 35 mg/m2 on days 1 and 8 of a 21-day cycle. GEMOX consisted of Gemcitabine IV 1,000 mg/m2 and Oxaliplatin 100 mg/m2 on days 1 and 15 of a 28-day cycle.
Procedures
Radiological tumor response data were retrieved from original radiological reports and there was no independent radiological imaging review. Assessment of tumor response was performed every 2 to 3 months using multi-detector computer tomography or magnetic resonance imaging. Biochemical tumor response was assessed according to changes in serum CA 19-9 after the beginning of Gemcitabine-based treatment. Such measurements took place every two to three months. Patients with baseline serum CA 19-9 ≤37 U/mL were considered to have a normal tumor marker level.
Predictor variables
We collected data on the following baseline patients’ characteristics: age, gender, ECOG performance status, Age-Adjusted Charlson Comorbidity Score (AACCS), body mass index (BMI), tumor site (head/neck vs. body/tail), number of metastatic sites, serum CA 19-9 before the start of Gemcitabine-based chemotherapy (U/mL), neutrophil-to-lymphocyte ratio (NLR) before the start of Gemcitabine-based chemotherapy, use of prior adjuvant Gemcitabine, number of previous lines of treatment for metastatic disease, duration of progression-free survival (PFS) interval (according to RECIST 1.1) while on FOLFIRINOX in the first-line setting, time interval from the last cycle of FOLFIRINOX to start of Gemcitabine-based chemotherapy, type of Gemcitabine-based chemotherapy, and deployment of granulocyte colony stimulating factors (G-CSF) during Gemcitabine-based chemotherapy. Patients for whom performance status data were not available had their performance inferred from the descriptions of patients’ capabilities found in the medical records. We also collected information regarding additional lines of treatment after failure of Gemcitabine-based chemotherapy, including the number of further lines of treatment and the chemotherapy regimens used.
Outcome variables
The co-primary outcomes of the study were the OS and the PFS of patients diagnosed with MPA and treated with Gemcitabine-based chemotherapy after progression on first-line FOLFIRINOX. As secondary outcomes, we assessed the radiological RR and the disease control rate (DCR) according to RECIST 1.1, the biochemical RR using the changes in CA 19-9 levels, and the toxicity profile. We assessed OS from the start of FOLFIRINOX (OS1) and from the start of Gemcitabine-based chemotherapy (OS2). OS was defined as the time from start of treatment (either FOLFIRINOX or Gemcitabine-based chemotherapy) to death from any cause. PFS was defined as the time from start of Gemcitabine-based chemotherapy to disease progression according to RECIST 1.1 or death (whatever occurred first). Patients were censored at the last follow-up visit in the absence of an event. DCR was defined as the proportion of patients experiencing objective response or stable disease at the first tumor response evaluation. We defined biochemical response as at least a 50% reduction in CA 19-9 level from baseline. Patients with normal levels of serum tumor markers at diagnosis were considered ineligible for this analysis. Toxicity was graded according to the Common Toxicity Criteria for Adverse Events version 4.0. The safety analysis included the assessment of treatment delays (any delay), need for dose reduction during treatment, treatment discontinuation (and its reasons), severe treatment-related toxicity (treatment-related complication mandating hospital admission) and treatment-related mortality. As an exploratory analysis, we compared the outcomes of patients treated with single-agent Gemcitabine with those of patients undergoing Gemcitabine-based polychemotherapy.
Statistical analysis
We used absolute values and ratios to describe the distribution of categorical variables and Fisher’s exact test to compare the distributions of such variables. We used median values and the interquartile ranges (IQR) to describe the distribution of numerical variables. We generated curves to describe time-to-event variables (OS and PFS) according to the Kaplan-Meier method. Survival curves were compared using the log-rank test. We used Cox proportional-hazard model to performed univariate analysis of OS and PFS. We selected variables with P value <0.25 in the univariate analysis for the multivariate model. As baseline NLR and serum CA 19-9 showed right-skewed distributions, for didactic purposes, we transformed these variables to the logarithmic base (Log10) in the regression model. Also, due to the relatively modest sample size, we used ECOG performance status (0–1 vs. 2) and number of previous lines of treatment (1 vs. ≥2) as dichotomous variables. We considered two-tailed P values <0.05 as statistically significant. Statistical analysis was performed with the software R Project version 3.4.0.
Results
Overall, 104 patients with MPA underwent treatment with FOLFIRINOX in the first-line setting in our institution. We identified 59 patients who received Gemcitabine-based chemotherapy after FOLFIRINOX. Seventeen patients were excluded for the following reasons: ECOG ≥3 (11 patients), no progression on FOLFIRINOX according to RECIST 1.1 (two patients) and treatment outside A.C. Camargo Cancer Center (four patients). As a result, 42 patients constitute the study population.
Table 1 depicts patients’ characteristics at the start of Gemcitabine-based chemotherapy. Median age was 59.0 years (IQR: 53.0–64.0). Twenty-five (59.5%) patients were male and most patients presented ECOG 0 (N=8; 19.0%) or ECOG 1 (N=21; 59.5%). Most primary tumors were located in the body or tail of the pancreas (N=27; 64.3%). The median CA 19-9 level was 997.0 U/mL (IQR: 218.1–3,177.0) and the median NLR was 4.0 (IQR: 2.1–5.8). Most patients (N=33; 78.6%) had Gemcitabine-based chemotherapy as second-line treatment.
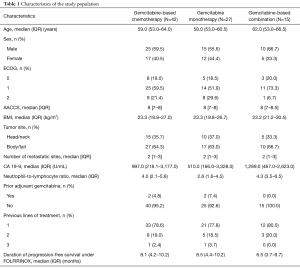
Full table
Treatments
In the first-line setting, patients were treated with FOLFIRINOX for a median of 12.0 cycles (IQR: 8.2–16.0 cycles). Median PFS under FOLFIRINOX treatment was 8.1 months (IQR: 4.2–10.2 months). All patients discontinued FOLFIRINOX due to radiological disease progression according to RECIST 1.1. The median time between the last cycle of FOLFIRINOX and the start of Gemcitabine-based chemotherapy was 1.2 months (IQR: 0.8–2.8 months).
Among 42 patients treated with Gemcitabine-based chemotherapy, 27 received treatment with single-agent Gemcitabine and 15 underwent treatment with Gemcitabine-based polychemotherapy. In the second group, eight patients were treated with Gemcitabine plus Nab-Paclitaxel, five patients with Gemcitabine plus Cisplatin and two patients with GEMOX. Overall, patients were submitted to a median of 3 cycles of chemotherapy (IQR: 3–4 cycles). The median number of cycles of single-agent Gemcitabine was 3 (IQR: 2.5–3). Conversely, individuals submitted to polychemotherapy underwent a median of 4 cycles of treatment (IQR: 3–6 cycles). Seven patients (16.7%) received decreased doses of chemotherapy from the start (14.8% for single-agent Gemcitabine vs. 20% for Gemcitabine-based combinations). Primary prophylaxis with G-CSF was not used by any patient. During the time span of the study, Nab-Paclitaxel was not promptly available in Brazil as this medication had to be imported. As a consequence, after progression on FOLFIRINOX, patients swiftly started treatment with Gemcitabine and Nab-Paclitaxel was incorporated without tumor progression after a median 31.5 days from the start of Gemcitabine (IQR: 21.0–46.2 days).
RR and DCR
Table 2 illustrates radiological and biochemical responses to Gemcitabine-based chemotherapy. Among 42 patients, only one patient (2.4%) experienced partial response according to RECIST 1.1. This patient was treated in second-line setting with Gemcitabine plus Nab-Paclitaxel after treatment with FOLFIRINOX for 2.2 months. DCR for all patients was 33.4%. Patients undergoing Gemcitabine-based polychemotherapy presented numerically higher DCR (46.7% vs. 25.9%; P=0.19). Besides, the attainment of disease control at the first radiological response assessment was associated with increased OS [hazard ratio (HR) =0.28; 95% CI, 0.12–0.64; P=0.002].
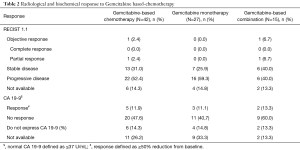
Full table
Five patients (11.9%) showed biochemical response to treatment. There was no significant difference in the percentage of patients achieving biochemical response according to the type of Gemcitabine-based chemotherapy (single-agent vs. combination; P=1.00).
Survival outcomes and prognostic factors
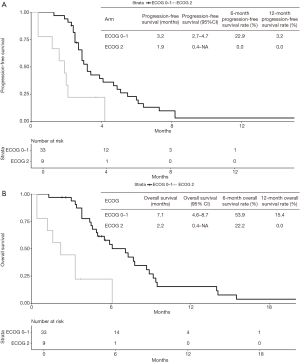
Median follow-up time was 20.8 months and four patients were lost to follow-up. At last follow-up, 40 patients (95.2%) had Gemcitabine-based chemotherapy permanently interrupted. Treatment was discontinued due to disease progression in 32 patients, due to clinical deterioration in five patients and due to limiting toxicity in three patients. Thirty-nine patients experienced death or disease progression. Median PFS from the start of Gemcitabine-based chemotherapy was 2.9 months (95% CI, 2.5–4.1 months) (Figure 1A). PFS rates at 3 and 6 months were 48.6% and 19.2%, respectively. At last follow-up, 35 patients were dead. Median OS from the start of Gemcitabine-based chemotherapy (OS2) was 5.5 months (95% CI, 3.8–7.3 months) (Figure 1B). OS rates at 6 and 12 months were 46.2% and 12.3%, respectively. Median OS from the start of FOLFIRINOX in the first-line setting (OS1) was 14.6 months (95% CI, 12.0–16.3 months). OS rates from the start of first-line chemotherapy at 12 and 18 months were 68.2% and 28.6%, respectively.
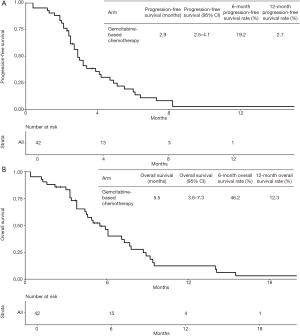
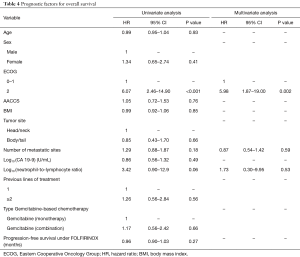
In the multivariate model, ECOG performance status was the only variable independently associated with PFS (ECOG 2: HR =2.97; 95% CI, 1.22–7.22; P=0.01) (Table 3). Indeed, median PFS for patients with ECOG 0–1 at start of Gemcitabine-based chemotherapy was 3.2 months (95% CI, 2.7–4.7 months), while patients with ECOG 2 presented median PFS of 1.9 months (95% CI, 0.4–NA months) (Figure 2A). For OS, again, ECOG performance status was the only independent prognostic factor (ECOG 2: HR =5.98; 95% CI: 1.87–19.0; P=0.002) (Table 4). Median OS for patients with ECOG 0–1 at the start of Gemcitabine was 7.1 months (95% CI, 4.6–8.7 months) and 2.2 months (95% CI, 0.4–NA months) for those with ECOG 2 (Figure 2B).
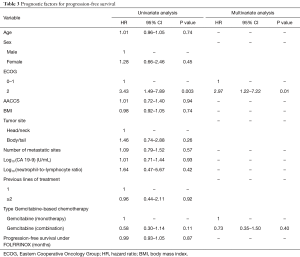
Full table
Full table
There were no statistically significant differences in OS or PFS according to the type of Gemcitabine-based chemotherapy performed (single-agents vs. combination) in multivariate analysis. We also compared survival outcomes of patients treated with single-agent Gemcitabine to those of patients treated with Gemcitabine plus Nab-Paclitaxel. Again, we did not observe statistically significant differences in PFS (HR =0.58, 95% CI, 0.25–1.31; P=0.19) or OS (HR =0.96, 95% CI, 0.38–2.40).
Toxicity
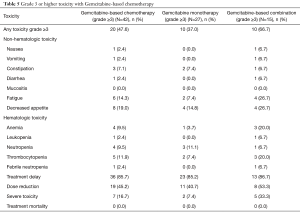
Table 5 describes the toxicity profile of Gemcitabine-based chemotherapy. Grade 3–5 toxicities were observed in 47.6% of patients. Figures were higher for patients undergoing treatment with polychemotherapy (66.7% vs. 37.0%; P=0.10). The most important grade 3–5 non-hematological toxicities were fatigue (14.3%) and decreased appetite (19.0%). The hematological toxicity was mild, with less than 10% incidence of grade 3–5 toxicities, except for thrombocytopenia (11.9%). Thirty-six patients (85.7%) experienced treatment delays and 19 patients (45.2%) needed chemotherapy dose reductions during the course of treatment. Severe toxicities were observed in seven patients (16.7%) and there were no treatment-related deaths.
Full table
Further treatments
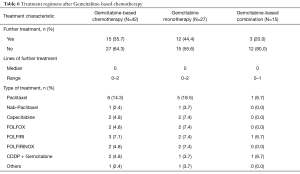
Table 6 describes the additional treatments undertaken after Gemcitabine failure. Fifteen patients (35.7%) were submitted to further treatments. Numerically, more patients treated with single-agent Gemcitabine underwent additional treatments (44.4% vs. 20.0%; P=0.18). The most commonly used regimen following progression on Gemcitabine was paclitaxel monotherapy.
Full table
Discussion
Chemotherapy plays an important role in the treatment of MPA, providing symptom palliation and benefits in OS (17). So far, the most encouraging results in the first-line setting have been achieved using the FOLFIRINOX regimen. In the pivotal ACCORD 11/PRODIGE 4 trial (2), the median OS for those treated with FOLFIRINOX was 11.1 months, and in a recently presented randomized phase II trial (SWOG S-1313) evaluating the role of recombinant human hyaluronidase, patients treated with modified FOLFIRINOX presented a median OS of 14.4 months. That is the longest survival ever reported for patients with MPA in a randomized clinical trial.
Nevertheless, virtually all patients treated with FOLFIRNOX will experience disease progression, and evaluation of the potential benefits and harms of further treatments is paramount. After Gemcitabine monotherapy, second-line treatments based on 5-fluorouracil have been established as standards of care for those with adequate performance status based in randomized phase III trials (4-7). However, there are no randomized trials evaluating the role of additional lines of treatment following FOLFIRINOX. Recently, researchers have made significant progress in the understanding of the molecular mechanisms underlying the pathogenesis of pancreatic cancer and impressive results were seen in subgroups of patients treated with Immunotherapy (18), NTKR inhibitors (19) and PARP inhibitors (20). Nevertheless, only a very small number of patients derive benefit from such targeted strategies and this highlights the necessity to further explore the role of available chemotherapeutic agents in MPA after disease progression on FOLFIRINOX.
In our study, despite the low objective RR, a significant proportion of patients experienced disease control when treated with Gemcitabine-based chemotherapy. Other studies have shown similar results with RRs ranging from 10% to 30% and DCRs ranging from 26% to 60% (8,10-16,21). We also showed that at 6 months, nearly 20% of patients treated with Gemcitabine-based chemotherapy are progression-free. Accordingly, other studies have shown PFS rates at 6 months ranging from 16% to 83% (8,15,21). That said, a subgroup of patients definitely benefits from Gemcitabine-based chemotherapy following disease progression on FOLFIRINOX.
Despite these results, chemotherapy clearly does not aid all patients and some might even experience significant treatment-related toxicities. Thus, patient selection is crucial in deciding whether or not to provide these patients further cytotoxic treatments. In this regard, we showed that performance status is an extremely important factor to bear in mind. Patients with ECOG performance status 2 had a median OS of only 2.2 months, significantly shorter than the median OS of those with ECOG performance status 0–1 (7.1 months). Other studies assessing the role of Gemcitabine-based chemotherapy in the second-line setting after FOLFIRINOX (21) or after other chemotherapeutic regimens (22,23) have also demonstrated the importance of using patients’ performance status to weight out potential benefits of chemotherapy. Moreover, in a recent large study evaluating factors associated with improved survival in the second-line treatment for advanced pancreatic adenocarcinoma, performance status proved to be the single most important factor predicting outcome (23). As a result, before deciding to offer patients further treatment after progression on FOLFIRINOX, a meticulous evaluation of patients’ clinical status must be performed.
Some studies have shown that other factors can predict benefits from Gemcitabine-based chemotherapy after FOLFIRINOX. In special, it has been suggested early progression on FOLFIRINOX is a harbinger of greater efficacy during second-line Gemcitabine-based chemotherapy (8,11,13). In our study, we could not replicate this finding, perhaps due to the modest sample size. But interestingly, the sole patient presenting radiological response to chemotherapy was one treated with Gemcitabine plus Nab-Paclitaxel after experiencing disease progression only 2.2 months after the start of FOLFIRINOX. Components of FOLFIRINOX and Gemcitabine (and Nab-Paclitaxel) present different mechanisms of action, theoretically rendering them non-cross-resistant. These data strengthen the role of Gemcitabine-based chemotherapy after early disease progression on FOLFIRINOX. However, care must be taken in interpreting the results of studies assessing the relationship between the duration of disease control with FOLFIRINOX and subsequently with Gemcitabine-based chemotherapy as some results are not statistically significant (described as trends) and large retrospective studies have not shown a significant correlation between these two outcomes (23).
Another way to assist in selecting patients for further treatment could rely on the molecular characteristics of the tumor. The expression of the human equilibrative nucleoside transporter-1 (hENT1) as assessed by immunohistochemistry has been shown to predict the benefit of adjuvant Gemcitabine after pancreatic cancer resection (24). Nonetheless, the results of studies assessing its role in the metastatic setting have been disappointing, as they have found poor correlation between hENT immunohistochemical expression and benefits from Gemcitabine (25,26). The lack of predictive capability may be associated with the use of different antibodies (27) and may be circumvented by deploying other techniques, such as RT-PCR (28). Moreover, recent data point to increased anti-cancer activity of Gemcitabine plus Nab-Paclitaxel in patients with tumors lacking class III β-tubulin as assessed by immunohistochemistry (29). These data pave the way to an optimal approach to select patients for Gemcitabine-based chemotherapy based on molecular features of the tumors and additional research is needed to fully describe the role of these and other potential markers of treatment efficacy.
After patients failing treatment with FOLFIRINOX have been properly selected to undergo Gemcitabine-based chemotherapy, there remains a doubt regarding the optimal regimen to be used. In our study, there was no significant difference in OS based on the type of Gemcitabine-based chemotherapy used (single-agent vs. polychemotherapy). Nevertheless, in the univariate analysis of PFS, there was a suggestion that polychemotherapy may achieve better results than single-agent Gemcitabine. Additionally, disease control ratio was numerically higher in patients treated with Gemcitabine-based combinations. Retrospective series evaluating the activity of single-agent Gemcitabine have shown objective RRs around 10.0% and DCRs ranging from 26.0% to 40.0% (10,11,21). These figures seem lower than the ones found in retrospective studies of Gemcitabine-based combos, especially those including Nab-Paclitaxel. Retrospective studies assessing the activity of Gemcitabine plus Nab-Paclitaxel after FOLFIRINOX have demonstrated RRs up to 30% and DCRs up to 60% (8,12,13,15,16).
Survival results also seem to be superior using Gemcitabine plus Nab-Paclitaxel. This combination has yielded median PFS times between 2.8 and 5.1 months and median OS times between 5.7 and 12.4 months (8,12,13,15,16). Also, the longest median OS attained so far in MPA (18 months) was seen in an observational study in which patients were treated sequentially with FOLFIRINOX and Gemcitabine plus Nab-Paclitaxel (8). These data, along with the increased activity of Gemcitabine plus Nab-Paclitaxel shown in the first-line setting (30), set this combination as a valuable option for those progressing on FOLFIRINOX.
Nonetheless, polychemotherapy is associated with increased toxicity. In our study, combined regimens presented increased incidence of severe side-effects. This is in line with the findings of randomized trials evaluating Gemcitabine-based combinations in the first-line setting (2,30) and with retrospective analyses showing that, after FOLFIRINOX, Gemcitabine plus Nab-Paclitaxel is associated with higher rates of grades 3–5 adverse events than single-agent Gemcitabine (13,21). Thus, the improved activity of this regimens comes at the expense of increased toxicity and this information is paramount when choosing chemotherapy regimens for those with worse clinical conditions, as they are prone to more severe toxicities.
Our study presents limitations. It is a retrospective study with a relatively modest sample size. Also, patients treated with Gemcitabine plus Nab-Paclitaxel presented at least some delay in starting Nab-Paclitaxel, possibly hampering outcomes in these patients. Nonetheless, our study portrays the outcomes of a homogenous cohort of patients treated in a single center, with detailed information. We believe our study adds significant information to the current knowledge, especially regarding the importance of performance status in selecting patients for second-line treatment after FOLFIRINOX and the benefits and harms of combination chemotherapy in this setting.
To summarize, some patients with MPA derive significant benefit from treatment with Gemcitabine-based chemotherapy following progression on FOLFIRINOX. Numerically, the benefit seems to be greatest using Gemcitabine-based polychemotherapy. Nevertheless, care should be taken when treating patients with combined regimens as there is an increased risk of toxicity. In this regard, we should use performance status to aid in treatment selection. Patients with ECOG performance 2 present short survival times in this setting. Were these patients to be treated, due to their limited survival outcomes and putatively increased risk of toxicity, we favor single-agent Gemcitabine over polychemotherapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the A.C. Camargo Cancer Center Internal Ethics Review Board (CAAE 88206718.7.0000.5432). The study was IRB approved for waiver of informed consent as it’s a retrospective study.
References
- Sirri E, Castro FA, Kieschke J, et al. Recent Trends in Survival of Patients with Pancreatic Cancer in Germany and the United States. Pancreas 2016;45:908-14. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Pelzer U, Schwaner I, Stieler J, et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: A phase III-study from the German CONKO-study group. Eur J Cancer 2011;47:1676-81. [Crossref] [PubMed]
- Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: Outcomes from the CONKO-003 Trial. J Clin Oncol 2014;32:2423-9. [Crossref] [PubMed]
- Gill S, Ko YJ, Cripps C, et al. PANCREOX: A randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 2016;34:3914-20. [Crossref] [PubMed]
- Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016;387:545-57. [Crossref] [PubMed]
- Portal A, Pernot S, Tougeron D, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer 2015;113:989-95. [Crossref] [PubMed]
- da Rocha Lino A, Abrahão CM, Brandão RM, et al. Role of gemcitabine as second-line therapy after progression on FOLFIRINOX in advanced pancreatic cancer: A retrospective analysis. J Gastrointest Oncol 2015;6:511-5. [PubMed]
- Gilabert M, Chanez B, Rho YS, et al. Evaluation of gemcitabine efficacy after the FOLFIRINOX regimen in patients with advanced pancreatic adenocarcinoma. Medicine (Baltimore) 2017;96. [Crossref] [PubMed]
- Sarabi M, Mais L, Oussaid N, et al. Use of gemcitabine as a second-line treatment following chemotherapy with folfirinox for metastatic pancreatic adenocarcinoma. Oncol Lett 2017;13:4917-24. [Crossref] [PubMed]
- Zhang Y, Hochster H, Stein S, et al. Gemcitabine plus nab-paclitaxel for advanced pancreatic cancer after first-line FOLFIRINOX: single institution retrospective review of efficacy and toxicity. Exp Hematol Oncol 2015;4:29. [Crossref] [PubMed]
- Nguyen KT, Kalyan A, Beasley HS, et al. Gemcitabine/nab-paclitaxel as second-line therapy following FOLFIRINOX in metastatic/advanced pancreatic cancer—retrospective analysis of response. J Gastrointest Oncol 2017;8:556-65. [Crossref] [PubMed]
- Bertocchi P, Abeni C, Meriggi F, et al. Gemcitabine plus Nab-Paclitaxel as second line and beyond for metastatic pancreatic cancer (MPC): A single institution retrospective analysis. Rev Recent Clin Trials 2015;10:142-5. [Crossref] [PubMed]
- El Rassy E, Assi T, El Karak F, et al. Could the combination of Nab-paclitaxel plus gemcitabine salvage metastatic pancreatic adenocarcinoma after folfirinox failure? A single institutional retrospective analysis. Clin Res Hepatol Gastroenterol 2017;41:e26-8. [Crossref] [PubMed]
- Dadi N, Stanley M, Shahda S, et al. Impact of Nab-Paclitaxel-based Second-line Chemotherapy in Metastatic Pancreatic Cancer. Anticancer Res 2017;37:5533-9. [PubMed]
- Sultana A, Smith CT, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 2007;25:2607-15. [Crossref] [PubMed]
- Le DT, Durham JN, Smith KN, et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409-13. [Crossref] [PubMed]
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Viaud J, Brac C, Artru P, et al. Gemcitabine as second-line chemotherapy after Folfirinox failure in advanced pancreatic adenocarcinoma: A retrospective study. Dig Liver Dis 2017;49:692-6. [Crossref] [PubMed]
- Caparello C, Vivaldi C, Fornaro L, et al. Second-line therapy for advanced pancreatic cancer : evaluation of prognostic factors and review of current literature. Future Oncol 2016;12:901-8. [Crossref] [PubMed]
- Vienot A, Beinse G, Louvet C, et al. Overall Survival Prediction and Usefulness of Second-Line Chemotherapy in Advanced Pancreatic Adenocarcinoma. J Natl Cancer Inst 2017;109. [Crossref] [PubMed]
- Bird NTE, Elmasry M, Jones R, et al. Immunohistochemical hENT1 expression as a prognostic biomarker in patients with resected pancreatic ductal adenocarcinoma undergoing adjuvant gemcitabine-based chemotherapy. Br J Surg 2017;104:328-36. [Crossref] [PubMed]
- Poplin E, Wasan H, Rolfe L, et al. Randomized, multicenter, phase II study of CO-101 versus gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma: Including a prospective evaluation of the role of hENT1 in gemcitabine or CO-101 sensitivity. J Clin Oncol 2013;31:4453-61. [Crossref] [PubMed]
- Ormanns S, Heinemann V, Raponi M, et al. Human equilibrative nucleoside transporter 1 is not predictive for gemcitabine efficacy in advanced pancreatic cancer: Translational results from the AIO-PK0104 phase III study with the clone SP120 rabbit antibody. Eur J Cancer 2014;50:1891-9. [Crossref] [PubMed]
- Caparello C, Meijer LL, Garajova I, et al. FOLFIRINOX and translational studies: Towards personalized therapy in pancreatic cancer. World J Gastroenterol 2016;22:6987-7005. [Crossref] [PubMed]
- Orlandi A, Calegari MA, Martini M, et al. Gemcitabine versus FOLFIRINOX in patients with advanced pancreatic adenocarcinoma hENT1-positive: everything was not too bad back when everything seemed worse. Clin Transl Oncol 2016;18:988-95. [Crossref] [PubMed]
- Kato A, Naiki-Ito A, Naitoh I, et al. The absence of class III β-tubulin is predictive of a favorable response to nab-paclitaxel and gemcitabine in patients with unresectable pancreatic ductal adenocarcinoma. Hum Pathol 2018;74:92-8. [Crossref] [PubMed]
- Wang XF, Huang WF, Nie J, et al. Toxicity of chemotherapy regimens in advanced and metastatic pancreatic cancer therapy: A network meta-analysis. J Cell Biochem 2018;119:5082-103. [Crossref] [PubMed]


