Anaemia and its effects on tumour regression grade and survival following chemotherapy in adenocarcinoma of the oesophagus
Introduction
The UK National Oesophago-Gastric Cancer Audit recorded 15,340 patient diagnoses of carcinoma between 2013–2015 (1). It is the eighth most common cancer and sixth most common cause of cancer mortality worldwide (2). Anaemia is highly prevalent in those suffering from cancer and this has been shown to be as a result of the cancer itself as well as due to the toxic effects of chemotherapy (3). A significant survival benefit for operable gastro-oesophageal adenocarcinoma has been shown by combining surgery and chemotherapy in randomized control trials (4). In the UK, neoadjuvant and adjuvant chemotherapy using the MAGIC regimen of ECF (epirubicin, cisplatin and 5-flurouricil) is the most common approach. This includes an accepted substitution of drugs within class (infusional 5-FU to oral capecitabine: ECX, and cisplatin to oxaliplatin: EOX) which maintains efficacy, whilst reducing platinum toxicity and aiding pyrimidine administration (5).
The histopathological response to the chemotherapy can be measured in the resection sample, and graded from 1–5 using the tumor regression grading (TRG) as described by Mandard (6) (Table 1). Oesophageal cancer response to chemotherapy can be broadly classed as chemo-sensitive (TRG 1–3) and chemo-resistant (TRG 4–5), which directly relates to prognosis (7,8) and is a significant predictor of disease free survival (6).
There is no literature studying the consequences of anaemia on histopathological response in chemotherapy for oesophageal cancer. However, the most relatable studies examined rectal carcinoma for histological response in chemo-radiotherapy. In one chemo-radiation study of 463 patients, tumour response (classified by T/N staging) found that patients with haemoglobin greater than 12 g/dL had a significant better tumour response and morphology compared to lower levels of haemoglobin (9). In chemo-radiotherapy for colorectal adenocarcinoma, it was demonstrated in a group of 490 patients that those patients with a haemoglobin over 9 g/dL had significantly better TRG [1–3] response (10). These studies show there is potential for anaemia to decrease tumour response in cancer therapy. However, no literature investigates tumour response in chemotherapy for oesophageal adenocarcinoma.
There is also no literature on the consequences of anaemia on survival outcomes in oesophageal cancer treated with neoadjuvant chemotherapy. Studies in gastric cancer alone investigated the association of low haemoglobin on outcomes in patients undergoing chemotherapy with 5-fluorouracil. They found that in those patients with a low haemoglobin (<10 g/dL) there was a significantly lower response rate to chemotherapy (measured by CT staging) as well as increased (disease progression and survival time (P<0.001) (11). This was also supported by a more recent study on the effects of anaemia in gastric cancer treated with chemotherapy (12).
Anaemia may lead to decreased oxygen levels in the tumour tissue, which is known as anaemic tissue hypoxia. When tissues undergo anaemic hypoxia, activation of a transcription factor called ‘hypoxia induced factor’ occurs. This factor then binds to hormone response elements on certain target genes activating a hypoxic response (13). These can then lead to alterations in angiogenesis (14), apoptosis (15), mutagenesis (13) as well as drug uptake and accumulation (14) which may hypothetically impact on tumour growth. Some cytotoxic agents require there to be adequate oxygen levels to produce their maximal cytotoxic effect. Therefore decreased oxygen, as seen in anaemic hypoxia, may directly cause reduced drug efficacy (15). Hypoxia can also cause chemo-resistance by genomic changes. For instance acidotic and apoptotic hypoxic selection pressures can lead to clonal production of resistant cells which may be more aggressive, leading to a worse prognosis (14).
The aim of this study is to establish whether anaemia impacts on prognosis as defined by overall survival and tumour response in those undergoing chemotherapy for oesophageal adenocarcinoma. We hypothesize that anaemia will reduce chemotherapy efficacy and tumour sensitivity (and thus tumour response) leading to a worse overall survival during chemotherapy for oesophageal adenocarcinoma.
Methods
Ethics
Ethical approval was granted for this study by the ethical committee for the Nottingham University Hospitals Clinical Audit and Evaluation office (reference 14-404C). Informed consent was obtained from all patients as part of the Nottingham University Hospitals tissue biobank and data was held in accordance with the Data Protection Act 1998 and in adherence to Caldicott principles.
Patients
This study used a prospectively compiled database of patients who underwent resection for histologically proven oesophageal adenocarcinoma at Nottingham University Hospitals NHS Trust, between August 2006 and February 2013.
Chemotherapy
In total 268 patients were included in this study. This cohort was administered ECF/ECX/EOX chemotherapy and underwent surgery on an intention to treat basis. All patients had available full blood counts at the time of each cycle. Other chemotherapy data recorded included dosage reductions, complication/toxicity, number of cycles and any cycle reductions. These were extracted from the hospitals’ ChemoCare database (CIS Oncology, Coventry, UK) with any missing data retrospectively identified and incorporated using Notis (Nottingham University Hospital Trust’s electronic record system).
Surgery
Surgery was undertaken by a team of surgeons each carrying out >20 cancer resections per year. These procedures were all carried out in a single specialist centre. Peri-operative care was in line with departmental protocols and therefore standardised.
The resected specimens were examined by histopathologists and designated a Mandard TRG and TMN stage. TRG was assessed by three gastrointestinal pathologists in a standard pathological exam of the clinical specimen, which is performed to the RCPath guidelines. A second pathologist then reviewed this during the upper gastrointestinal cancer multi-disciplinary team meeting, and a response confirmed. If there is significant discrepancy between pathologists, independent blinded review by a third takes place for a final decision. TRG 1–3 patients were classed as ‘responders’ to chemotherapy whilst those with TRG 4–5 were classed as ‘non-responders’.
Anaemia
Anaemia data was retrieved electronically from Nottingham and its associated referral hospitals. Full blood counts were retrospectively recorded, identifying haemoglobin before commencement of chemotherapy and before the start of each individual treatment cycle. Haemoglobin (g/dL) and mean corpuscular volume were recorded. Pre-chemotherapy anaemia and nadir anytime anaemia (during each of the cycles of chemotherapy) were then determined.
WHO criteria for anaemia were used to categorise anaemia (<12 g/dL in females and <13 g/dL in males) and non-anaemic patients. Anaemia severity was also classified by the following criteria: severe (<8 g/dL), moderate (8–10.9 g/dL) in both males and females with mild (11–12.9 g/dL), low normal (13–14.9 g/dL), high normal (>15 g/dL) in males and mild (11–1.9 g/dL), low normal (12–13.9 g/dL) and high normal (>14 g/dL) in females.
Outcome measures
Overall survival was defined as date of diagnosis until date of death. TRG was marker for histological tumour response.
Statistical analysis
SPSS (version 22, IBM) was used to perform statistical analysis, with a P<0.05 used to determine statistical significance. Patient characteristics were recorded and analysed by percentages along with the median and its ranges. The effect of anaemia against TRG was analysed using a Chi-squared (χ2) test. To demonstrate survival analysis, Kaplan-Meier survival curves were used.
A multivariate Cox regression analysis was used to calculate HRs with 95% confidence intervals to determine potential confounders and the size of their effect on the survival analysis. This allows for the production of an adjusted hazard ratio of this analysis.
Results
Patient characteristics
The study identified 268 patients of which 84% (n=226) were male and 16% (n=42) were female. The median age at the time of operation for each sex was 67 years for females and 65 years for males. The majority of cancers (n=130) were located in the gastro-oesophageal junction or the lower third of the oesophagus (n=79). A majority (n=228) underwent the ECX chemotherapy regime with a minority administered the ECF (n=30) and EOX (n=10) regimens. Patient demographics are displayed in Table 2 and histological locations demonstrated in Table 3.
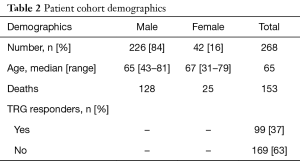
Full table
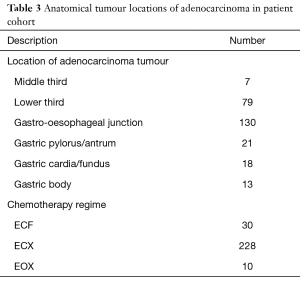
Full table
Anaemia
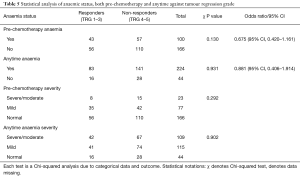
Before each participant underwent their first cycle of chemotherapy 37% (n=100) were anaemic; whilst during and after cycles it was found that at 84% (n=224) continued to have or develop anaemia. Of those in the pre-chemotherapy anaemic cohort, 29% (n=77) had mild anaemia with 9% (n=23) moderate-severe anaemia, whilst 62% (n=166) were not anaemic. Of those in the anytime anaemic cohort, 43% (n=115) had mild anaemia and 41% (n=109) had moderate-severe anaemia (Table 4).
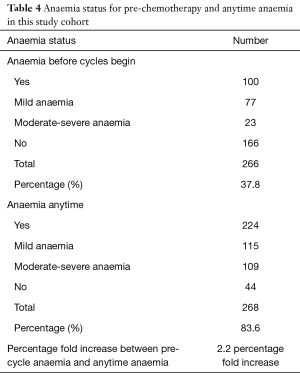
Full table
Survival outcomes
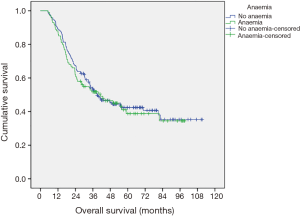
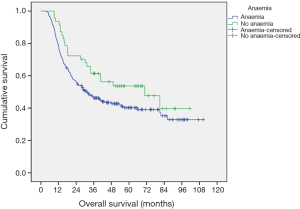
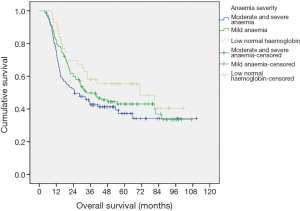
There is no significant association between pre-chemotherapy anaemia and overall survival (P=0.770) (Figure 1), nor was a statistical significance found between those who suffered from anytime anaemia and overall survival (P=0.07) (Figure 2). Analysis into severity of anytime anaemia, as shown by normal, mild and moderate/severe haemoglobin, found a statistical association (P=0.048), between the increasing severity of anaemia and worsening overall survival (Figure 3). When comparing moderate and severe anaemia versus normal haemoglobin levels, a more significant association (P=0.026) is observed (Figure 4). Therefore, it can be concluded that anaemia reduces overall survival.

Cox regression analysis allows us to take into account potential confounders such as TRG, stage of tumour, comorbidities, and alterations in chemotherapy cycles, dosage reductions, location of tumour and involved margins. Our analysis shows that only TRG and cancer staging are significant confounders and thus are included in the model. This subsequent model demonstrates that anytime anaemia is more significantly associated with overall survival (HR 1.735, 95% CI, 1.050–2.867, P=0.032).
More importantly, when cox regression is performed on severity of anaemia the link between severity and overall survival is more significant (HR 0.718, 95% CI, 0.559–0.923, P=0.010). This indicates that the more severe each patient’s anaemia, the greater the likelihood of a negative effect on the patient’s overall survival. Therefore, we can conclude that anytime anaemia and its increasing severity reduces overall survival.
Histopathological tumour response
Of the 268 patients 63% (n=169) were non-responders (TRG 4–5) with the other 27% (n=99) responding to the chemotherapy regimen (TRG 1–3). There were 100 pre-chemotherapy anaemic patients of whom 43% were classed as TRG ‘responders’ and 57% TRG ‘non-responders’. Of the 166 who were not anaemic pre-chemotherapy 34% were TRG ‘responders’ and 66% were TRG ‘non-responders’. No statistically significant association between pre-chemotherapy anaemia and TRG response was found (OR 0.675, 95% CI, 0.420–1.161, P=0.130). Analysing those who developed anaemia during chemotherapy cycles against TRG also found no significant association (OR 0.881, 95% CI, 0.406–1.914, P=0.931). Here, of the 224 who were anaemic at anytime, 37% were TRG ‘responders’ and 63% TRG ‘non-responders’ compared to the non-anaemic cohort (n=44) with 36% were ‘responders’ and 64% ‘non-responders’. There was no statistically significant association between severity of pre-chemotherapy anaemia (P=0.292) and anytime anaemia (P=0.902) with TRG (Table 5).
Full table
Discussion
This study demonstrated that those patients who suffered from anaemia during neoadjuvant MAGIC style chemotherapy for oesophageal adenocarcinoma had a worse prognosis with reduced overall survival. The impact of anaemia on chemotherapy response (as measured by TRG) was investigated as a potential reason for the decreased survival. In this large group of patients, no statistically significant association was observed between anaemia and TRG, suggesting that the impact of anaemia on survival is not related to chemotherapy response.
Response to chemotherapy
Although there appears to be a link between anaemia and tumour response with radiotherapy treatment, associations with chemotherapy and anaemia are less well described. All participants in this study were treated with similar chemotherapy regimens whose main mechanisms against cell proliferation are by direct crosslinking of DNA strands, not through indirect formation of free radicals. The platinum agents such as cisplatin and oxaliplatin work by crosslinking DNA strands at adjacent guanine molecules. The anthracyclines, such as epirubicin, also function by intercalating within DNA and by stopping topoisomerase II from allowing the unzipping of the DNA helix whilst fluorouracil inhibits the synthesis of nucleic acids (16). Therefore, these mechanisms may not be altered by anaemia. Conversely, one of epirubicin’s other mechanisms requires oxygen for free radical production and so could have reduced efficacy. Further in-vitro research into how these drugs may be affected under hypoxic conditions have led to the hypotheses that fluorouracil efficacy could be reduced due to reduced intracellular nucleotides during hypoxia (15). However, conflicting literature on cisplatin has found that some studies show enhanced toxicity, no changes or decreased efficacy under low oxygen conditions. These results seem to be determined by the cell line used to investigate and so cannot be reliably extrapolated to the clinical setting (15). This is unlike radiotherapy in which it has been shown that, when atmospheric oxygen is reduced to less than 25–30 mmHg, radio-sensitivity drastically reduces (17). Therefore, although anaemic hypoxia may lead to a number of alterations in cell behaviour and potentially decreasing efficacy of chemotherapy, our results suggest that it is not enough to individually alter the TRG.
Tumours may be inherently responsive or non-responsive. Of particular relevance is a recent study of 129 patients with adenocarcinoma of the oesophagus by the OCCAMS consortium using whole genome sequencing. It demonstrated that oesophageal cancers could be grouped according to their mutational signatures, which relate to therapeutic outcomes (18). It has also been shown by other studies that genetic make up may be directly involved in therapeutic tumour response (19). Therefore, it appears that inherent genetic makeup has a greater effect on tumour response to chemotherapy than any influence from anaemia.
Markers of tumour response
The histological assessment of TRG is subjective and thus has the potential for inter- and intra-observer sensitivity. However, as a standardised review procedure is adhered to in this study, the use of histopathological TRG as the marker of tumour response to chemotherapy is an accepted and validated measure.
Since TRG does not account for nodal involvement and therefore only represents the response of the primary tumour, it may not fully reflect the true prognosis. Both TRG and nodal involvement are independently associated with worse disease-free survival. For instance one study found that those with no TRG response but with nodal down-staging were significantly more likely to have increased disease-free survival compared to no nodal down-staging (20). TRG also does not account for the fact that chemotherapy helps in the systemic reduction of micro-metastases which helps to lead to decrease recurrence. The majority of oesophageal cancer patients still die from metastasis and indeed it has been found that even when tumours are staged as N0, later analysis can find evidence of metastasis (21). Therefore, all the benefits of chemotherapy are not localised in the primary tumour and thus TRG may not be a fully representative endpoint.
Anaemia’s effect upon survival
Survival analysis of pre-chemotherapy or anytime anaemia found no significant association with overall survival. However, when anaemia is categorised into severity, a statistically significant association is found. Therefore, it is apparent that as severity of anaemia increases there is a decrease in overall survival. Cox regression demonstrated that both anytime anaemia and anytime anaemia severity decreased overall survival with an increased statistical association observed.
Due to the lack of association found between pre-chemotherapy anaemia and survival, but significant associations found between anytime anaemia and survival, this timing indicates a possible association in patients with chemotherapy induced anaemia.
There are no studies that examine anaemia in adenocarcinoma of the oesophagus during chemotherapy and its effect on survival. This is unlike the extensive chemo-radiotherapy literature which show reduced survival outcomes (22,23). The most relatable studies focus on gastric cancer treated by platinum-based chemotherapy or combinations of paclitaxel, platinum and/or 5-FU (11,12). Both of these show how anaemia is associated with decreased survival outcomes. However, despite the potential similarities, this study cohort is based on a different chemotherapy regime and a different type of upper gastro-intestinal carcinoma.
Anaemia is associated with poor survival outcomes for many cancers (23,24). There are many hypotheses as to how anaemia may affect the outcome of chemotherapy. Since this study has shown that anaemia does not affect chemotherapy efficacy in a way that alters TRG it can only be assumed that it may in other ways that are not represented by TRG. It has been well documented that tissue hypoxia caused by anaemia can lead to induction of ‘hypoxia induced factor’. This may further stimulate angiogenesis of micro-vessels, disrupt cellular adhesions increasing risk of micro-metastases as well as altering genes that may lead to more aggressive cells or stimulate oncogenes (13,24). Therefore, anaemia may indicate a more severe disease, which could become more aggressive during and after chemotherapy. Although a lot of this research is produced in the laboratory setting, more clinical data has been published recently. It has been shown in oesophageal cancer that even mild anemia increases micrometastases and angiogenesis (25). Anaemia is also associated with poorer performance and functional status as well as increase comorbidities of which are independently prognostic in treatment of oesophageal cancers (26,27). Finally, anaemia has been shown to increase the risk of perioperative complications and postoperative mortality. These include cardiac events, sepsis and thromboembolism as well as respiratory difficulties. All of these lead to an increased length of hospital stay and reduced quality of life (28). Many patients with severe anaemia may have undergone blood transfusions depending on local trust guidelines and clinical features. There is still conflicting evidence that suggests that although raising haemoglobin levels increases survival in those undergoing chemoradiotherapy for oesophageal cancer it may also increase the risk of disease progression due to immune modulation (29,30).
The Cox regression model became statistically more significant when adjusted for tumour stage (the most strongly predictive marker in the prognosis of esophageal cancer), suggesting that as well an independent marker of worse survival anytime anaemia severity is maybe more prominent or its survival effect more marked in advanced disease. We can postulate that this effect maybe due to carcinomatosis, occult bleeding, haemolysis or bone marrow infiltrate.
This is the only study that investigates the effect of anaemia on chemotherapy in oesophageal cancer and although anaemia was not shown to alter TRG it has been shown to reduce overall survival in these patients. This cannot be explained fully, however as discussed there are many potential explanations that may play a role in this finding.
Conclusions
In conclusion, no association was found between anaemia and tumour regression grade after neoadjuvant chemotherapy and resection for oesophageal adenocarcinoma, although a reduced overall survival was observed in those with anaemia of worsening severity during chemotherapy treatment.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: Ethical approval was granted for this study by the ethical committee for the Nottingham University Hospitals Clinical Audit and Evaluation office (reference 14-404C). Informed consent was obtained from all patients as part of the Nottingham University Hospitals tissue biobank and data was held in accordance with the Data Protection Act 1998 and in adherence to Caldicott principles.
References
- RCS. National Oesophago-Gastric Cancer Audit. 2016.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Dicato M, Plawny L, Diederich M. Anemia in cancer. Ann Oncol 2010;21 Suppl 7:vii167-72. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [Crossref] [PubMed]
- Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer 1994;73:2680-6. [Crossref] [PubMed]
- Fareed KR, Ilyas M, Kaye PV, et al. Tumour regression grade (TRG) analyses in patients with resectable gastro-oesophageal adenocarcinomas treated with platinum-based neoadjuvant chemotherapy. Histopathology 2009;55:399-406. [Crossref] [PubMed]
- Fareed KR, Al-Attar A, Soomro IN, et al. Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastro-oesophageal cancer treated with neoadjuvant chemotherapy. Br J Cancer 2010;102:1600-7. [Crossref] [PubMed]
- Khan AA, Klonizakis M, Shabaan A, et al. Association between pretreatment haemoglobin levels and morphometric characteristics of the tumour, response to neoadjuvant treatment and long-term outcomes in patients with locally advanced rectal cancers. Colorectal Dis 2013;15:1232-7. [Crossref] [PubMed]
- Lee SD, Park JW, Park KS, et al. Influence of anemia on tumor response to preoperative chemoradiotherapy for locally advanced rectal cancer. Int J Colorectal Dis 2009;24:1451-8. [Crossref] [PubMed]
- Park SH, Lee J, Lee SH, et al. Anemia is the strongest prognostic factor for outcomes of 5-fluorouracil-based first-line chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol 2006;57:91-6. [Crossref] [PubMed]
- Ye X, Liu J, Chen Y, et al. The impact of hemoglobin level and transfusion on the outcomes of chemotherapy in gastric cancer patients. Int J Clin Exp Med 2015;8:4228-35. [PubMed]
- Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. J Mol Med (Berl) 2007;85:1301-7. [Crossref] [PubMed]
- Shannon AM, Bouchier-Hayes DJ, Condron CM, et al. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev 2003;29:297-307. [Crossref] [PubMed]
- Wouters A, Pauwels B, Lardon F, et al. Review: implications of in vitro research on the effect of radiotherapy and chemotherapy under hypoxic conditions. Oncologist 2007;12:690-712. [Crossref] [PubMed]
- Rang HP, Dale MM, Ritter JM, et al. Moore. Pharmacology. 5th ed. Bath: Elsevier, 2003.
- Vaupel P, Thews O, Hoeckel M. Treatment resistance of solid tumors: role of hypoxia and anemia. Med Oncol 2001;18:243-59. [Crossref] [PubMed]
- Secrier M, Li X, de Silva N, et al. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet 2016;48:1131-41. [Crossref] [PubMed]
- Tao CJ, Lin G, Xu YP, et al. Predicting the Response of Neoadjuvant Therapy for Patients with Esophageal Carcinoma: an In-depth Literature Review. J Cancer 2015;6:1179-86. [Crossref] [PubMed]
- Noble F, Nolan L, Bateman AC, et al. Refining pathological evaluation of neoadjuvant therapy for adenocarcinoma of the esophagus. World J Gastroenterol 2013;19:9282-93. [Crossref] [PubMed]
- Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 2014;32:2983-90. [Crossref] [PubMed]
- Rades D, Lang S, Schild SE, et al. Prognostic value of haemoglobin levels during concurrent radio-chemotherapy in the treatment of oesophageal cancer. Clin Oncol (R Coll Radiol) 2006;18:139-44. [Crossref] [PubMed]
- Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med 2004;116 Suppl 7A:11S-26S. [Crossref] [PubMed]
- Clarke H, Pallister CJ. The impact of anaemia on outcome in cancer. Clin Lab Haematol 2005;27:1-13. [Crossref] [PubMed]
- Krzystek-Korpacka M, Matusiewicz M, Diakowska D, et al. Even a mild anemia is related to tumor aggressiveness mediated by angiogenic factors. Exp Oncol 2009;31:52-6. [PubMed]
- Kandaz M, Ertekin MV, Bilici M. Retrospective analysis of patients with esophageal cancer treated with radiotherapy and/or chemoradiotherapy. Tumori 2012;98:445-50. [Crossref] [PubMed]
- Clavier JB, Antoni D, Atlani D, et al. Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis Esophagus 2014;27:560-7. [Crossref] [PubMed]
- Hans GA, Jones N. Preoperative anaemia. Contin Educ Anaesth Crit Care Pain 2013;13:71-4. [Crossref]
- Tuan J, Ha TC, Pan S, et al. Prognostic significance of blood transfusion and anaemia on survival in stage IIIA/B/C and IVA oesophageal cancers treated with chemoradiotherapy. J Radiat Oncol 2014;3:167-77. [Crossref]
- Kosumi K, Baba Y, Harada K, et al. Perioperative Blood Transfusion, Age at Surgery, and Prognosis in a Database of 526 Upper Gastrointestinal Cancers. Dig Surg 2015;32:445-53. [Crossref] [PubMed]



