Impact of intensity modulated radiation therapy on survival in anal cancer
Introduction
The incidence of anal cancer (AC) has been rising at an average annual rate of 2.2% over the last 10 years. Concomitantly, death rates have also been increasing, on average, at 3.2% each year between 2004–2013 (1). Historically, the management of this disease was abdominoperineal resection (APR). However, in 1974, Nigro et al. introduced sphincter preserving chemoradiation (CRT) as a new treatment paradigm (2). Subsequent studies have since confirmed the efficacy of this treatment approach, which has led to the establishment of CRT as the first line treatment for AC (3-7). This paradigm shift from surgical management to CRT has yielded excellent clinical outcomes with 5-year colostomy rate and overall survival (OS) of 12% and 78%, respectively, as reported in the long-term update of RTOG 98-11 (8).
Although CRT for AC has demonstrated excellent response rates, this treatment option is associated with significant toxicities. Patients can experience painful moist skin desquamation, diarrhea, and a significant decline in bone marrow reserve. In the initial report of RTOG 98-11, the rate of acute non-hematologic grade 3 or 4 toxicity was 74% in both mitomycin/5-fluorouracil (5-FU) and cisplatin/5-FU groups (9). More recently, the ACT II trial found similar grades 3–4 adverse events of 71% and 72%, in the mitomycin/5-FU and cisplatin/5-FU groups, respectively (10).
One potential method to reduce radiotherapy (RT) related toxicity, is to utilize intensity modulated radiation therapy (IMRT) since it affords the opportunity to deliver highly conformal doses to the tumor while minimizing dose to adjacent organs. IMRT is particularly suited for the treatment of AC because of the abundance of adjacent organs at risk such as small intestine, rectosigmoid colon, bladder, skin, bone marrow, and external genitalia. RTOG 0529, the first multi-institutional trial examining the role of IMRT in AC, found that IMRT was associated with significant sparing of acute grade 2+ hematologic, grade 3+ dermatologic and gastrointestinal toxicity (11). However, whether such reductions in toxicity can potentially translate into an improvement in survival, remains to be seen.
The present study was designed to evaluate the difference in survival of patients with AC treated with IMRT versus non-IMRT based CRT. We utilized the National Cancer Data Base (NCDB), a large, prospectively acquired database that includes approximately 70% of newly diagnosed cancer patients treated at over 1,500 facilities in the United States.
Methods
Patient selection
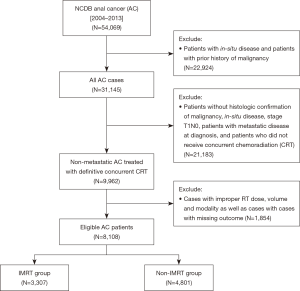
The NCDB 2014 Participant User File (PUF) for AC was obtained for this analysis. This included patients with AC diagnosed between 2004 and 2013. The database was queried for patients with non-metastatic AC patients. In order to be eligible, patients were required to have histologic confirmation of malignancy. We excluded patients that had in-situ disease, non-squamous histology, metastatic disease at diagnosis, and patients with history of prior malignancy. We further excluded patients who did not receive definitive concurrent CRT, who received inappropriate RT doses or volumes, and cases with missing outcomes. Patients were excluded if they were treated with radiosurgery, Gamma Knife, brachytherapy, radium, and radioisotopes. Patient demographics, socioeconomic status, disease characteristics, treatment details, and treatment outcomes were available for analysis. Patients were stratified into two groups: patients receiving IMRT based CRT and patients who received non-IMRT based CRT (Figure 1).
Patient demographics
Patient’s age at diagnosis, gender, race, type of health insurance, geographic location, education, median income quartile, treatment facility type were available for analysis. Geographic location was determined by the zip code of the patient’s residence recorded at the time of diagnosis. This was then classified and compared as metropolitan, urban, or rural location. Level of education for each patient was inferred by using the patient’s zip code at the time of diagnosis and with the aid of US census data, determined the number of adults in the patient’s zip code who did not graduate from high school. Patient level of income was estimated by matching the patient’s zip code to the median household income for the area of residence derived from US Census data. Treatment facility was categorized as academic/research center (post-graduate medical education training program and participation in cancer clinical research), which includes National Cancer Institute (NCI) designated comprehensive cancer center, or non-academic which includes community cancer program (more than 100 but ≤500 of new annual cancer cases) and comprehensive community cancer program (more than 500 new annual cancer cases). Charlson-Deyo score was used as a surrogate marker for patient co-morbidities. Charlson-Deyo score was categorized as 0, 1, or 2, and above to indicate increasing levels of comorbid conditions (12).
Disease characteristics
The following disease related variables were evaluated: diagnosis year, American Joint Cancer Committee (AJCC) clinical tumor and nodal stage, lymphovascular space invasion and tumor grade. Patients were staged based on the AJCC staging edition in use during the year in which the case was diagnosed.
Treatment details
Patients were eligible if they were treated with concurrent CRT. Chemotherapy agent (multi-agent versus single-agent), RT dose, and RT modality (IMRT vs. other) were also evaluated. Patients were excluded if they received inappropriate RT volume (outside the pelvis), non-standard RT dose (<44 or >70 Gy), or if they were unable to complete the prescribed course of RT. Patients were stratified in the IMRT group if their RT modality was IMRT, an external beam technique that was required to be clearly stated in the patient record. The remaining patients were stratified into the non-IMRT group. The non-IMRT group included patients that received 3D conformal RT (3D-CRT) and RT not otherwise specified (RT-NOS). 3D-CRT was defined as an external beam technique using multiple, fixed portals shaped to conform to a target volume. Again, 3D-CRT was required to be clearly stated in the patient record in order to be categorized as 3D-CRT. RT-NOS was categorized as patients who received RT using an external beam therapy machine with mixed energy photons and/or electrons.
Outcome
The primary outcome of this study was OS—defined as time from diagnosis to time of death or last follow-up.
Statistical analysis
All statistical analyses were conducted using SAS 9.4 (Cary, NC, USA). Univariate associations between each variable and the two study cohorts (IMRT vs. non-IMRT) were calculated using the χ2 test for categorical covariates and ANOVA for numerical covariates. The univariate analysis (UVA) between each covariate of interest and OS was assessed using Cox proportional hazard model and log-rank test. A multivariable Cox proportional hazard model for OS was fit using the backward selection method and a removal criterion of 0.20. Hazard ratios (HR) with associated 95% confidence interval (CI) were generated for each covariate and the outcome. Kaplan-Meier (KM) survival analysis was performed to generate OS curves comparing the outcomes of the two cohorts.
Propensity score (PS) analysis was implemented to account for the differences in patient demographics, tumor characteristics, and treatment details between both groups (13). A multinomial logistic regression model was created to estimate the propensity of a patient being in the IMRT or non-IMRT cohort. Variables included in the PS model were those hypothesized to be associated with OS (14). IMRT patients were matched 1:1 with non-IMRT patients using a greedy matching algorithm. Effectiveness of matching was evaluated by calculating the standardized differences of the covariates between treatment groups (15,16). After PS matching was applied, the effect of RT modality in the matched sample was recalculated using a Cox proportional hazard model. Adjusted KM curves were then regenerated to compare the outcomes of the two groups.
Results
The initial query of the NCDB 2014 anal cancer database resulted in 54,069 cases. After applying the aforementioned inclusion and exclusion criteria, there were a total of 8,108 eligible patients as depicted in Figure 1. Patients were then stratified based on the radiation modality: IMRT (n=3,307; 40.8%) and non-IMRT (n=4,801; 59.2%).
Patient characteristics
There were 5,368 (66.2%) female and 2,740 (33.8%) male patients. There were 4,545 (56.0%) of patients with stage II disease, and 3,563 (44%) with stage III disease. The majority of patients (n=6,879, 84.8%) were treated with multi-agent chemotherapy. The median and mean age at diagnosis was 57 and 58.29 years, respectively. The median and mean RT dose were 54 and 54.01 Gy, respectively. The median and mean duration of RT was 47.0 and 49.5 days, respectively. The median follow-up time for all patients was 54.4 months.
IMRT usage
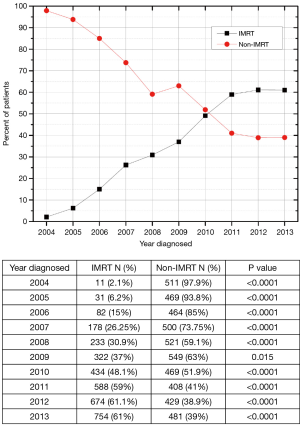
Between 2004 and 2007, there were 2,246 patients diagnosed with AC who received concurrent CRT. In that time period, 86.6% (n=1,944) of patients received non-IMRT and 13.4% (n=302) received IMRT. For the 2,528 AC cases diagnosed between 2008 and 2010, 60.9% (n=1,539) and 39.1% (n=989) received non-IMRT and IMRT, respectively. Next, for the 3,334 patients diagnosed with AC between 2011–2013, 39.5% (n=1,318) and 60.5% (n=2,016) of patients were treated with non-IMRT and IMRT, respectively. Figure S1 plots the annual IMRT usage trend nationwide. This shows an increasing use of IMRT from 2004 to 2013. For the year 2013, 61% of patients received IMRT based CRT.
UVA
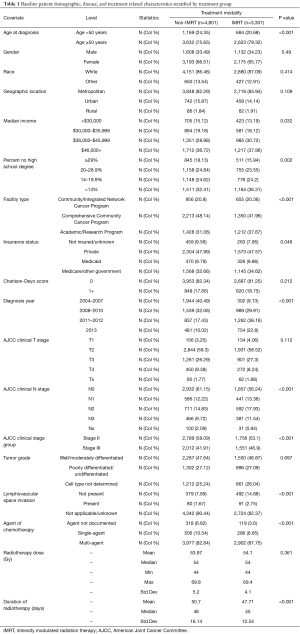
Table 1 demonstrates the baseline demographic, disease characteristics, and treatment details between the two patient groups. The two groups were well balanced except for patients in the IMRT group were statistically (all P<0.05) more likely to be older at diagnosis (age ≥50 years 79.3% vs. 75.7%), have Medicare or other government insurance (34.6% vs. 32.6%), reside in areas with median annual household income of $36,000–$45,999 (30.7% vs. 29.0%) and in areas with <14% no high school degree (36.3% vs. 32.4%), be treated at an Academic/Research Program (37.7% vs. 31.1%), diagnosed with clinical N2 disease (17.9% vs. 14.8%) and overall stage III (46.9% vs. 41.9%), have no evidence of lymphovascular space invasion (14.9% vs. 7.9%), treated with multi-agent chemotherapy (87.8% vs. 82.8%), diagnosed in 2011–2012 (38.2% vs. 17.4%), and complete RT in a shorter duration (median 45 vs. 48 days).
Full table
Multivariate analysis (MVA)
Unadjusted MVA for OS showed that IMRT (HR 0.84, 95% CI: 0.75–0.93; P<0.001), male gender (HR 1.65, 95% CI: 1.50–1.81; P<0.005), presence of lymphovascular space invasion (HR 2.07, 95% CI: 1.49–2.88; P<0.001), academic/research treatment facility (HR 0.86, 95% CI: 0.75–0.98; P=0.021), Medicare insurance (HR 1.40, 95% CI: 1.18–1.66; P<0.001), rural patient location (HR 1.44, 95% CI:1.05–1.98; P=0.023), receipt of single agent chemotherapy (HR 1.39, CI: 1.11–1.73; P=0.004), age ≥50 years (HR 1.19, 95% CI: 1.05–1.34; P=0.006), Charlson-Deyo score ≥1 (HR 1.50, 95% CI: 1.35–1.67; P<0.001), advanced AJCC clinical nodal stage (HR 1.63, 95% CI: 1.41–1.89; P<0.001), clinical tumor stage (HR 3.26, 95% CI: 2.36–4.51; P<0.001), and total RT dose (HR 1.00, 95% CI: 1.00–1.00; P=0.01) were also statistically significant.
Treatment outcome
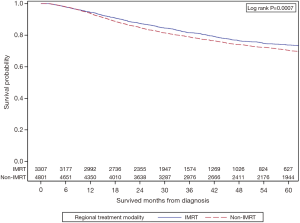
Figure 2 shows the unadjusted KM survival analysis stratified by treatment group. The 5-year OS of the IMRT group was 73.8% vs. 70.4% in the non-IMRT group (P=0.0007).
PS analysis
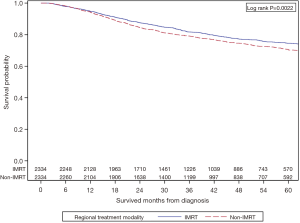
All variables that were statistically significant on UVA between the two groups and MVA for OS were incorporated into the PS matching. Age, gender, race, education, median income quartile, geographic location, treatment facility type, health insurance status, diagnosis year, Charlson-Deyo score, tumor grade, lymphovascular space invasion, agent of chemotherapy, AJCC clinical tumor and nodal stage, and RT dose were all included in the balancing. This resulted in 2,334 IMRT patients being matched to similar 2,334 non-IMRT patients with standardized differences across all variables <0.1 (Table 2). After application of PS matching, Cox-proportional model for OS showed that the IMRT group had superior survival with HR 0.83 (95% CI: 0.74–0.94, P=0.002). The adjusted KM survival analysis comparing the two cohorts is shown in Figure 3. This shows that IMRT is associated with improved OS at 2 years 87.6% vs. 84.5%, and at 5 years 74.6% vs. 70.5%, P=0.0022. Median OS was not reached in both groups.

Full table
Discussion
The use of advanced RT techniques to minimize inadvertent dose to adjacent organs has been proven to have a dosimetric advantage in AC (17,18), however, whether such dosimetric advantages translate into clinical gains in survival remains largely unknown. The large, prospectively acquired, multi-institutional database such as the NCDB potentially allows for large statistical power needed to detect a small survival difference when evaluating the impact of RT technique on clinical outcomes. Our investigation of 8,108 non-metastatic AC patients shows that treatment with IMRT has statistically significant superior OS when compared to similar patients treated with non-IMRT based CRT. This survival advantage persisted despite the implementation of statistical techniques to eliminate selection bias. To our knowledge, this is the first study with the primary end point of OS that evaluates the impact of IMRT in AC. Moreover, our analysis provides insight into the nationwide practice patterns and usage of IMRT (Figure S1). We observed that for new cases diagnosed in 2013, 61% of patients were treated with IMRT.
The results of our study are directly comparable to phase III, randomized, multi-institutional clinical trials including: ACCORD-03 (19), RTOG 98-11 (8), and ACT II (10) where patients were treated with non-IMRT based CRT. In the present study, the 5-year of 70.5% in the non-IMRT group is similar to the 71% 5-year OS of the non-induction, standard RT dose group in the ACCORD-03 trial (19). Next, the 5-year OS of the mitomycin group in RTOG 98-11 and the no maintenance mitomycin group of ACT II study were 78.3% and 79%, respectively. This difference in survival is likely due to tumor and treatment heterogeneity amongst the studies. Only 30% of patients in the mitomycin group of RTOG 98-11 and 31% of patients in maintenance mitomycin group of ACT II trial had nodal metastases, compared to the non-IMRT group of the present study which included 41% of patients with N1–N3 disease. Furthermore, 100% of patients in the aforementioned randomized trials received multi-agent chemotherapy, whereas, 86% of patients in the non-IMRT group received multi-agent chemotherapy. In the present analysis, the type of chemotherapy (mitomycin vs. cisplatin) could not be included in the analysis since this information is not captured in the NCDB. Additionally, our series reflects a broader, non-clinical trial, patient population representing approximately 70% of cancer diagnoses annually.
The rationale for using IMRT for AC was proposed after the publication of RTOG 98-11, where dose escalation to 59.4 Gy for T2–T4 lesions was suggested in order to improve local control. This led to grades 3–4 non-hematologic toxicity rates of 73% in the mitomycin group. Similarly, in the ACT II trial, 71% of patients in the no maintenance mitomycin group, despite using a lower RT dose of 50.4 Gy, suffered from grade 3 or 4 adverse events. Since patients in both of these trials were treated with non-IMRT, it is possible that their clinical outcomes are hampered by treatment related toxicities. In an effort to reduce chemotherapy related toxicities, there have been randomized clinical trials attempting to substitute mitomycin for cisplatin, however, at the time of publication, mitomycin with 5-FU used concurrently with RT, still remains the standard of care. While mitomycin and 5-FU have remained as the standard first line systemic agents, the dose, volume, and technique of RT utilized in AC has evolved over time. Since chemotherapy related toxicities could not be mitigated any further, attention was turned towards minimizing the side effects related to RT in order to maximize the therapeutic ratio. To that end, RTOG 0529, a phase II multi-institutional trial evaluating the role of IMRT in AC, showed that IMRT was associated with significant sparing of acute grade 2+ hematologic, grade 3+ dermatologic and gastrointestinal toxicity (11). Notably, the adoption of IMRT amongst radiation oncologists was associated with a learning curve: 81% of patients in RTOG 0529 required planning revisions and 46% required multiple resubmissions. In order to avoid reduction in local control by so called “marginal miss”, IMRT requires understanding the complexities associated with target and nodal basin delineation. Due to the challenges associated with adopting IMRT into practice, one could postulate that IMRT could potentially lead to inferior outcomes. However, the results of this study, even after PS matching, directly contradict this hypothesis and align with previously published single institutional data (20).
The survival gains from using IMRT are hypothesis generating and this could be due to a combination of multiple factors: decreased treatment related acute side effects resulting in fewer treatment breaks, reduction in myelosuppression, and decreased long-term events. Due to accelerated repopulation, interruption or prolongation of the prescribed RT course has been linked to inferior clinical outcomes not only in AC (21) but also in HNSCC (22), cervical cancer (23), and bladder cancer (24). Since IMRT has been shown to decrease acute side effects, it has the potential to reduce treatment breaks and in turn lead to improved tumor control. Moreover, fewer interruptions and reduction in acute toxicities can lead to completion of the prescribed chemotherapy course, as previously shown in a SEER analysis where AC patients treated with IMRT were more likely to receive ≥2 cycles of mitomycin- or cisplatin-based chemotherapy (25). In the present analysis, both groups received the same median RT dose of 54 Gy, however, the duration of RT was shorter with IMRT (mean 47.7 versus 50.7 days, median 45 versus 48 days, and standard deviation 10.5 versus 16.1 days; P<0.001). Next, combining mitomycin with RT, both of which are known to be toxic insults to the bone marrow, can collectively lead to severe hematologic toxicities and profound myelosuppression. Such levels of bone marrow toxicity, particularly in a patient population that not infrequently suffers from chronic human immunodeficiency virus (HIV) infection and possibly acquired immune deficiency syndrome (AIDS), may increase mortality from opportunistic infections. While majority of AC patients do not harbor HIV, any myelotoxic agents have the potential to attenuate the tumoricidal response emanating from the innate and adaptive immune system, which can subsequently lead to worse tumor control. Minimizing the amount of pelvic bone marrow exposed to RT has the potential to preserve the immune response both in immunocompetent and immunocompromised patients. Lastly, late toxicity including small intestinal obstructions, fistulas, ulcerations, radiation proctitis, and hip fractures, although do not directly contribute to mortality, could theoretically limit patients’ ability to receive salvage treatments (including surgery, systemic therapy and re-irradiation) and in turn lead to inferior long-term survival outcomes. A potential confounder for the observation of improved survival in the IMRT group is that since patients in the IMRT group were more likely to be treated in the recent era [2010–2013], they were more likely to be staged with positron emission tomography (PET) and hence our results could be biased by stage migration. However, approximately 85–90% of AC patients present with non-metastatic disease (26) and a CT chest/abdomen/pelvis provides similar sensitivity for detecting hepatic and pulmonary metastases. Furthermore, for the non-metastatic cases, implementation of statistical tools such as PS matching, minimized the underlying patient demographic, tumor related, and treatment level heterogeneities between the two cohorts.
There are a few pertinent limitations of our analysis. Toxicity information (GI, skin, hematologic, etc.), chemotherapy agents used (mitomycin versus cisplatin), and HIV status were unavailable in the NCDB and hence could not be evaluated. Next, NCDB does not include progression free survival (PFS) or colostomy free survival (CFS) data and hence we could not compare the PFS/CFS of our patients against prior published randomized clinical trials. Majority of patients were missing the status of lymphovascular space invasion (87.2%).
Conclusions
To the best of our knowledge, this is the largest study to date that evaluates the impact of IMRT on OS in patients AC. Our investigation shows that IMRT based concurrent CRT for non-metastatic AC is associated with improved survival when compared to similar patients treated with non-IMRT based therapy. The survival benefit for IMRT persisted despite the implementation of statistical methods to minimize selection bias. Furthermore, we observed that only 61% of patients diagnosed with AC in the United States in 2013 received IMRT—likely due to lack of prospective, randomized data supporting its use. In the absence of randomized evidence, our analysis might provide additional support for increasing the use of IMRT for patients with new diagnosis AC managed with concurrent CRT.
Acknowledgements
We would like to thank the American College of Surgeons Commission on Cancer and the American Cancer Society for access to the data that enabled this analysis.
Funding: Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: Since this research study utilized the National Cancer Database (NCDB) which is a multi-institutional, de-identified cancer registry, informed consent or a review board approval is not necessary or applicable.
References
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review. Based on November 2015 SEER data submission. April 2016 ed. Bethesda, MD National Cancer Institute; 2016.
- Nigro ND, Vaitkevicius VK, Considine B Jr. Combined therapy for cancer of the anal canal: a preliminary report. 1974. Dis Colon Rectum 1993;36:709-11. [Crossref] [PubMed]
- Sischy B, Doggett RL, Krall JM, et al. Definitive irradiation and chemotherapy for radiosensitization in management of anal carcinoma: interim report on Radiation Therapy Oncology Group study no. 8314. J Natl Cancer Inst 1989;81:850-6. [Crossref] [PubMed]
- Cummings BJ, Keane TJ, O'Sullivan B, et al. Epidermoid anal cancer: treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys 1991;21:1115-25. [Crossref] [PubMed]
- Martenson JA, Lipsitz SR, Lefkopoulou M, et al. Results of combined modality therapy for patients with anal cancer (E7283). An Eastern Cooperative Oncology Group study. Cancer 1995;76:1731-6. [Crossref] [PubMed]
- Doci R, Zucali R, La Monica G, et al. Primary chemoradiation therapy with fluorouracil and cisplatin for cancer of the anus: results in 35 consecutive patients. J Clin Oncol 1996;14:3121-5. [Crossref] [PubMed]
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [Crossref] [PubMed]
- Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol 2012;30:4344-51. [Crossref] [PubMed]
- Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008;299:1914-21. [Crossref] [PubMed]
- James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2×2 factorial trial. Lancet Oncol 2013;14:516-24. [Crossref] [PubMed]
- Kachnic LA, Winter K, Myerson RJ, et al. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 2013;86:27-33. [Crossref] [PubMed]
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. [Crossref] [PubMed]
- Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res 2011;46:399-424. [Crossref] [PubMed]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- Imbens GW. The role of the propensity score in estimating dose-response functions. Biometrika 2000;87:706-10. [Crossref]
- Austin PC. The relative ability of different propensity score methods to balance measured covariates between treated and untreated subjects in observational studies. Med Decis Making 2009;29:661-77. [Crossref] [PubMed]
- Chen YJ, Liu A, Tsai PT, et al. Organ sparing by conformal avoidance intensity-modulated radiation therapy for anal cancer: dosimetric evaluation of coverage of pelvis and inguinal/femoral nodes. Int J Radiat Oncol Biol Phys 2005;63:274-81. [Crossref] [PubMed]
- Milano MT, Jani AB, Farrey KJ, et al. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys 2005;63:354-61. [Crossref] [PubMed]
- Peiffert D, Tournier-Rangeard L, Gerard JP, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol 2012;30:1941-8. [Crossref] [PubMed]
- Dasgupta T, Rothenstein D, Chou JF, et al. Intensity-modulated radiotherapy vs. conventional radiotherapy in the treatment of anal squamous cell carcinoma: a propensity score analysis. Radiother Oncol 2013;107:189-94. [Crossref] [PubMed]
- Ben-Josef E, Moughan J, Ajani JA, et al. Impact of overall treatment time on survival and local control in patients with anal cancer: a pooled data analysis of Radiation Therapy Oncology Group trials 87-04 and 98-11. J Clin Oncol 2010;28:5061-6. [Crossref] [PubMed]
- Bentzen SM. Repopulation in radiation oncology: perspectives of clinical research. Int J Radiat Biol 2003;79:581-5. [Crossref] [PubMed]
- Fyles A, Keane TJ, Barton M, et al. The effect of treatment duration in the local control of cervix cancer. Radiother Oncol 1992;25:273-9. [Crossref] [PubMed]
- Maciejewski B, Majewski S. Dose fractionation and tumour repopulation in radiotherapy for bladder cancer. Radiother Oncol 1991;21:163-70. [Crossref] [PubMed]
- Pollom EL, Wang G, Harris JP, et al. The Impact of Intensity Modulated Radiation Therapy on Hospitalization Outcomes in the SEER-Medicare Population With Anal Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys 2017;98:177-85. [Crossref] [PubMed]
- Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2007. National Cancer Institute 2010.