Unresectable metastatic colorectal cancer patient cured with cetuximab-based chemotherapy: a case report with new molecular insights
Introduction
Cetuximab-based chemotherapy is a standard of care for patients with RAS wild-type metastatic colorectal cancer (CRC) (1). However, it is not used as curative intent and the disease generally relapses or get progressive. Here we report the case of patient who remains in complete remission nearly 10 years after cetuximab discontinuation.
Case presentation
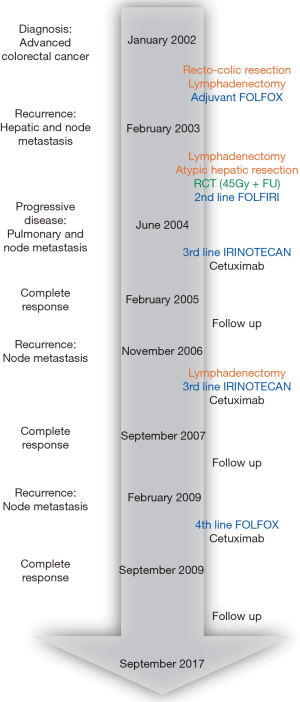
A 20-year-old Asian man, without a family history of cancer, was diagnosed in January 2002 with a locally advanced CRC. The evolution of his disease and treatments is summarized in Figure 1. In February 2002, he underwent a rectosigmoid resection and a lymphadenectomy of the aorto-iliac bifurcation. The pathological analysis found a poorly to moderately differentiated adenocarcinoma; sixteen nodes were collected, of which eight were metastatic with capsular rupture for five of them. The tumor was classified as pT4, N2, M0 with positive mesosigmoid sections. Twelve cycles of adjuvant chemotherapy with 5-fluorouracil (5FU) and oxaliplatin (i.e., FOLFOX) were administered from March to August 2002. At the end of treatment, carcinoembryonic antigen (CEA) and computerized tomography scan (CT scan) were normal.
In February 2003, the CT scan showed evidence of a hepatic and node recurrence: there was one 2 centimeters (cm) liver lesion (segment VII) and one adenopathy along the left iliac axis measuring 2.8 cm with node infiltration up to the origin of the external iliac artery. The patient underwent a left ilio-inguinal lymphadenectomy with epiplo-plasty, and an atypic hepatic resection. The pathological analysis reported several metastatic lymph nodes and assessed the diagnosis of hepatic recurrence. Then, the patient had a radiochemotherapy from April to May 2003: 45 Gy on the left inguino-iliac volume, with additional 10 Gy to the tumor bed. Concurrent chemotherapy with 5FU as a continuous infusion was performed throughout the radiation treatment. Second-line chemotherapy with 5FU and irinotecan (i.e., FOLFIRI) was subsequently scheduled but discontinued after 4 cycles because of severe digestive side effects.
In June 2004, a second metastatic recurrence with lung metastasis and retroperitoneal lymph nodes was diagnosed on abdominal pain. Epidermal growth factor receptor (EGFR) expression was assessed in the recto-sigmoid tumor and in the node metastases and its expression was high. A third-line treatment with cetuximab was started in August 2004 at 400 mg/m2 for the first injection, then 250 mg/m2 every week (qw), associated with irinotecan 180 mg/m2 every 2 weeks (q2w). Irinotecan was responsible for grade III diarrhea and was lowered to a 150 mg/m2 dosing. Complete radiological and biological response was obtained in February 2005 and the treatment was stopped at this date, after 12 courses of cetuximab and irinotecan.
In November 2006, a disease progression of the retrocaval and lombo-aortic nodes was observed. Irinotecan (150 mg/m2 q2w) and cetuximab (250 mg/m2 qw) were started again from January 2007 using. After 5 cycles, the patient achieved a partial response, with complete regression of lung metastasis. In May 2007, a lombo-aortic and illiac lymphadenectomy was then performed. The pathological analysis found node metastases from colon cancer. CT-scan and CEA were normalized, and chemotherapy was discontinued after 3 post-operative courses in September 2007.
In February 2009, the patient was diagnosed with a fourth recurrence located in lombo aortic and pelvic nodes only. A FOLFOX regimen combined with cetuximab (250 mg/m2 qw) was started from March 2009. After 5 cycles, there as evidence of partial response, and the treatment was continued with LV5FU2. By September 2009, the patient achieved complete response and treatment was stopped.
The last evaluation with clinical examination, CT scan and CEA dosing performed in September 2017 found no evidence of recurrence.
Extensive molecular characterization
An extensive molecular characterization of the patient’s cancer was performed given its rare and dramatic sensibility to EGFR-targeted therapy. We performed whole exome sequencing from fresh frozen tumor sample and adjacent normal tissue. We also assessed the microsatellite status of the tumor cells using Pentaplex panel. Finally, we determined the copy number variation of tumor cells of the primary tumor and the metastatic iliac lymph node by genotyping 250,000 single nucleotide polymorphisms (SNPs) on tumor and normal DNA using GeneChip Human Mapping 250K Sty Array (Affymetrix, Santa-Clara, CA, USA).
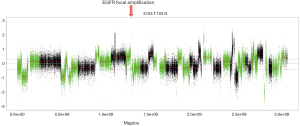
The patient tumor was found KRAS, NRAS, PIK3CA and BRAF wild-type with a microsatellite-stable (MSS) phenotype. Extended SNPs genotyping revealed that EGFR gene was highly amplified (more than 70 copies) (Figure 2). No difference in copy number variation was observed between the primary tumor and the iliac lymph node resected two years later.
Searching for a familial predisposition to cancer, the patient was found to carry a germline monoallelic inactivating MUTYH mutation at codon 156 (rs762307622, W156*, ExAC frequency in non-Finnish Europeans =0%); no mismatch repair gene germline mutation was found. Interestingly, following loss of the wild-type allele in tumor cells the mutation of the MUTYH gene became somatically homozygous. This biallelic MUTYH tumor mutation was associated with a mutational signature characterized by C > A single base substitutions and 164 non-silent single nucleotide variations (SNV).
Discussion
Here we report the case of a 20-year-old man who experienced a long-term complete response to cetuximab-based chemotherapy. The extensive characterization of his tumor revealed a high level of EGFR amplification, no mutation in KRAS, NRAS, PIK3CA and BRAF genes, but a monoallelic MUTYH germline mutation associated with an additional MUTYH inactivating mutation in tumor cells.
To the best of our knowledge, this is the first report of a nearly ten-years lasting complete response to cetuximab in a patient with metastatic CRC, even if several cases of complete responses to anti-EGFR therapy have been reported. Notably, Boudrias-Dalle et al. described the case of a patient with metastatic CRC who showed a complete response two years after panitumumab discontinuation; however no translational research for such a response was reported (2).
In 2004 cetuximab was demonstrated as clinically efficient in patients with CRC that expresses EGFR in both monotherapy and combination with irinotecan (3,4), given the rational for the use of cetuximab in our patient. EGFR expression using immunohistochemistry and EGFR gene copy number were initially suggested as potential biomarkers predictive for the efficacy of cetuximab (5). However, Lièvre et al. demonstrated that activating mutation in KRAS gene is the major mechanism of resistance to anti-EGFR antibodies, leading to the development of extended RAS mutational analysis (1,6). Nevertheless, it is worthy to note that an increased EGFR copy number was found in 3 patients among KRAS-wild type patients of Lièvre’s cohort and that it was associated with an objective tumor response to cetuximab (P=0.04). Similarly, Yen et al. showed that EGFR overexpression remained predictor of clinical response among KRAS wild-type patients (7), maybe because of an enhanced ADCC (Antibody-Dependent Cell-mediated Cytotoxicity) (8). The complete long-term response observed in our patient might be due to both the high-level amplification of EGFR and the absence of RAS mutation.
Our patient was found to carry a monoallelic mutation of MUTYH with an additional inactivation of the second allele by loss of heterozygoty in tumor cells. MUTYH is a DNA glycosylase involved in the repair of oxidative damage and has a role in base-excision repair system. Only a few data are published about monoallelic MUTYH germline mutation carriers. Notably there is no data about sensitivity of MUTYH-associated CRC to anticancer treatments. Biallelic loss-of-function MUTYH mutations predispose to familial CRC [i.e., MUTYH association polyposis (MAP)] through somatic G:C-T:A transversions in the adenomatous polyposis coli (APC) suppressive tumor gene (9). Lefevre et al. found that biallelic loss-of-function of MUTYH can lead to a loss of function of MLH1 and a microsatellite instability (MSI) cancer phenotype (10). Moreover c.34G > T KRAS mutation is highly associated with MUTYH-associated CRC, through G:C-T:A transversions in KRAS exon 2 (11,12). In accordance to this work, we found that MUTYH-associated cancers are defined by a specific mutational signature characterized by an enrichment of C > A transversions and relatively high mutational load (also inferior to mutational burden of MMR-deficient tumors (13-15). Interestingly, our patient’s tumor displayed a homozygous MUTYH mutation without KRAS or MLH1 mutation, while he was carrying a monoallelic MUTYH mutation. To our knowledge this loss of heterozygoty phenomenon has never been reported in MUTYH monoallelic carriers. Although the literature is dramatically poor about the chemosensitivity and the immunogenicity of MUTYH-mutated CRC, we might hypothesize in the case of our patient that the elevated mutational burden participated to the impressive long-lasting remission through immune-mediated response.
Conclusions
We report the case of a young patient diagnosed with a metastatic CRC more than fifteen years ago who might have been cured by cetuximab-based chemotherapy, as this treatment was discontinued more than 8 years ago. Overexpression of EGFR might be the reason of his dramatic response to cetuximab. Plus, while little is known the clinical impact of MUTYH mutations, it may have contributed to such a chemosensitivity. Our report paves the way for further researches on both MUTYH-associated cancers chemosensitivity and immunogenicity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
References
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. [Crossref] [PubMed]
- Boudrias-Dalle E, Cloutier M, Harvey M, et al. Durable complete remission following anti-EGFR antibodies in recurrent metastatic colorectal cancer. J Oncol Pharm Pract 2017. [PubMed]
- Saltz LB, Meropol NJ, Loehrer PJ Sr, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 2004;22:1201-8. [Crossref] [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [Crossref] [PubMed]
- Moroni M, Veronese S, Benvenuti S, et al. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol 2005;6:279-86. [Crossref] [PubMed]
- Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 2006;66:3992-5. [Crossref] [PubMed]
- Yen LC, Uen YH, Wu DC, et al. Activating KRAS mutations and overexpression of epidermal growth factor receptor as independent predictors in metastatic colorectal cancer patients treated with cetuximab. Ann Surg 2010;251:254-60. [Crossref] [PubMed]
- Perkins G, Pilati C, Blons H, et al. Beyond KRAS status and response to anti-EGFR therapy in metastatic colorectal cancer. Pharmacogenomics 2014;15:1043-52. [Crossref] [PubMed]
- Al-Tassan N, Chmiel NH, Maynard J, et al. Inherited variants of MYH associated with somatic G:C-->T:A mutations in colorectal tumors. Nat Genet 2002;30:227-32. [Crossref] [PubMed]
- Lefevre JH, Colas C, Coulet F, et al. MYH biallelic mutation can inactivate the two genetic pathways of colorectal cancer by APC or MLH1 transversions. Fam Cancer 2010;9:589-94. [Crossref] [PubMed]
- Jones S, Lambert S, Williams GT, et al. Increased frequency of the k-ras G12C mutation in MYH polyposis colorectal adenomas. Br J Cancer 2004;90:1591-3. [Crossref] [PubMed]
- Aimé A, Coulet F, Lefevre JH, et al. Somatic c.34G>T KRAS mutation: a new prescreening test for MUTYH-associated polyposis? Cancer Genet 2015;208:390-5. [Crossref] [PubMed]
- Germano G, Lamba S, Rospo G, et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 2017;552:116-20. [PubMed]
- Timmermann B, Kerick M, Roehr C, et al. Somatic mutation profiles of MSI and MSS colorectal cancer identified by whole exome next generation sequencing and bioinformatics analysis. PLoS One 2010;5. [Crossref] [PubMed]
- Pilati C, Shinde J, Alexandrov LB, et al. Mutational signature analysis identifies MUTYH deficiency in colorectal cancers and adrenocortical carcinomas. J Pathol 2017;242:10-5. [Crossref] [PubMed]