Outcome and factors associated with aborted cytoreduction for peritoneal carcinomatosis
Introduction
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) has improved the outcome for patients with peritoneal carcinomatosis (PC) from gastrointestinal malignancies (1,2). Cytoreduction involves the removal of all macroscopic disease within the abdominal cavity and often necessitates omentectomy, multi-visceral resection and complex peritonectomy procedures. Intraperitoneal chemotherapy is administered to eradicate residual microscopic disease (3). The burden of disease, as measured by the peritoneal cancer index (PCI), and the completeness of cytoreduction score (CCR) are significant determinants of outcome after CRS/HIPEC (4-6).
The successful completion of CRS/HIPEC is dependent on several factors, notably tumor grade, histology and primary tumor location (7). Pre-operative cross sectional imaging provides anatomic detail of the distribution of disease within the abdominal cavity but may under represent the exact burden of disease (8). Unfortunately, approximately 20% of patients who undergo exploration are found to have advanced disease precluding successful completion of CRS/HIPEC (9,10).
The negative impact on patients with PC who undergo aborted CRS procedures is potentially immense. Aborted cytoreduction procedures are associated with potential morbidity, unnecessarily prolongs recovery and may delay the initiation or continuation of systemic chemotherapy. The outcome of patients who are found to have significant disease and undergo aborted CRS procedures is ill-defined. The purpose of this study is to examine the outcomes of patients who undergo aborted CRS procedures and identify factors associated with aborted CRS in a newly established peritoneal surface malignancy program.
Methods
We performed a retrospective review of patients who were referred for management of peritoneal surface malignancies from December 2011 to February 2016. Patients were considered for CRS/HIPEC if they had a good performance status (ECOG 0/1), no evidence of extraperitoneal disease as evidenced on computed tomography (CT) or magnetic resonance imaging (MRI) and were able to tolerate a major abdominal operation. Patients were discussed in multidisciplinary tumor board prior to consideration for CRS/HIPEC. Approval for this study was obtained from the University of Tennessee Health Science Center and St Jude Children’s Research Hospital Institutional Review Boards.
At the time of exploration, the PCI score was calculated at the completion of adhesiolysis and prior to CRS/HIPEC (11). HIPEC was performed via a closed technique at the completion of cytoreduction. HIPEC was performed with mitomycin-C (40 mg at 42 ℃for 90 minutes) or oxaliplatin (200 mg at 42 ℃for 60 minutes) for appendiceal and colorectal pathology and cisplatin (100 mg/m2 at 42 ℃for 60 minutes) for ovarian, mesothelioma and desmoplastic small round cell tumor (DSRCT) pathologies. The adequacy of surgical debulking was defined by the CCR score (12). Gastrointestinal continuity, stoma creation (when necessary), and drain placement were performed at the completion of HIPEC.
Patient demographics, clinicopathologic data, and outcomes were collected in a prospectively maintained database. Detailed operative characteristics were recorded including procedures performed, PCI score, type and dose of chemotherapeutic agent used, duration of HIPEC, length of surgery, estimated blood loss (EBL), CCR score, need for intra-operative transfusion, extent of visceral resection, and number of anastomoses performed. Complications were graded according to the Clavien-Dindo classification schema (13).
Factors associated with aborted CRS were further analyzed including the distribution of disease and palliative procedures performed. Patients who underwent CRS/HIPEC were compared to those who underwent aborted CRS. Recurrence was defined as radiographic evidence of recurrent disease any time after CRS/HIPEC. Overall survival (OS) was defined as the duration from the date of CRS/HIPEC or aborted CRS to death or last follow up. For all outcomes, patients were censored at the time of most recent follow-up or death at the time of data collection. Categorical variables were summarized using percentages and compared using Chi-squared analysis. Continuous variables were summarized using the mean with standard deviation (SD) and analyzed with an independent, 2 tailed t-test. All P values were based on 2-tailed statistical analysis and a P value <0.05 was considered indicative of statistical significance. OS was compared between the two groups using the Kaplan-Meier method with the log rank test. All statistical analysis was performed using SPSS software, version 24 (IBM Corporation, Armonk, NY, USA).
Results
During the study period, 74 patients underwent evaluation for peritoneal surface malignancy and were scheduled for CRS/HIPEC. Of these, 51 patients (69%) underwent complete CRS/HIPEC and 23 patients (31%) underwent aborted CRS procedures. There were no significant differences between CRS/HIPEC and aborted CRS groups with respect to basic demographics including age, gender, race, or prior treatment (Table 1). Primary tumor histology differed between the groups as more patients with appendiceal primaries were able to undergo successful CRS/HIPEC, while a higher percentage of patients with colorectal pathology underwent aborted CRS (P=0.02).
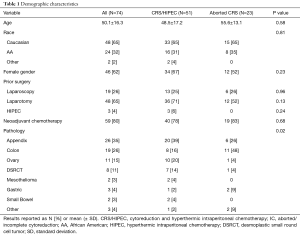
Full table
The PCI score was higher for patients who underwent aborted CRS procedures while those able to undergo CRS/HIPEC had a lower PCI score (26.1±9.9 vs. 16.2±10.5, P=0.001). Patients who were able to undergo complete CRS/HIPEC had significantly longer operative times, higher blood loss, were more likely to require transfusion, undergo multi-visceral resection, require an anastomosis and undergo a diverting ostomy as portion of their procedure (Table 2). There was no difference in hospital length of stay between groups.
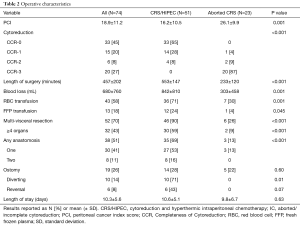
Full table
Patients who underwent CRS/HIPEC had a higher overall complication rate compared to those who underwent aborted procedures, although not statistically significant (43% vs. 22%, P=0.08). There was no difference in minor (Clavien-Dindo 1–2) or major (Clavien-Dindo 3–5) morbidity between groups (Table 3). Approximately one-quarter of patients in the CRS/HIPEC group required readmission within 30 days of operation.
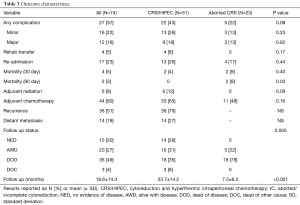
Full table
Adjuvant systemic chemotherapy was administered to 65% of patients in the CRS/HIPEC group while only 48% of patients in the aborted CRS group were treated with palliative systemic chemotherapy. Adjuvant whole abdominal radiation was administered to 6 patients with DSRCT which is part of the consolidative treatment protocol for this rare pathology at St Jude Children’s Research Hospital. Thirty-eight patients (75%) in the CRS/HIPEC group developed recurrence of disease with 14 (27%) developing distant disease (Table 3).
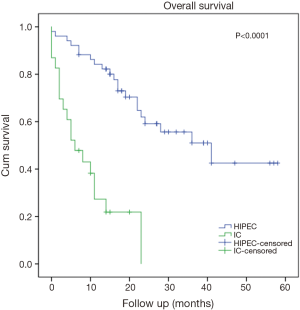
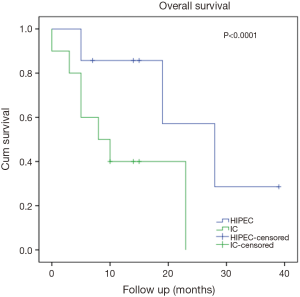
There was no difference in 30-day mortality between groups (Table 3). OS for all patients with PC evaluated for CRS/HIPEC was 22.8±3.0 months. The 1-, 3- and 5-year OS rates were 84%, 51% and 43% for CRS/HIPEC and were 27%, 0% and 0% for those who underwent aborted CRS. OS was 41.0±10.4 months for CRS/HIPEC versus 6.0±2.3 months for the aborted CRS group (P<0.0001, Figure 1). Patients with an appendiceal and a colorectal primary who underwent CRS/HIPEC had a significantly better outcome than those who underwent aborted CRS procedures (median not reached, >38 vs. 6±5.4 months, P<0.0001, Figure 2 and 28.0±7.5 vs. 8.0±4.0 months, P<0.0001, Figure 3, respectively). On multivariable analysis, PCI score (P<0.0001) and colorectal primary (P=0.014) were independent predictors of aborted CRS. PCI score (P=0.002) was the only factor significantly associated with OS (Table 4). With a median follow up of 15 months (range, 0–58 months), 28% of patients who underwent CRS/HIPEC had no evidence of disease, 31% were alive with disease and 35% had died of disease. In contrast, 21% of the aborted CRS groups were alive with disease and 78% had died of progressive disease.
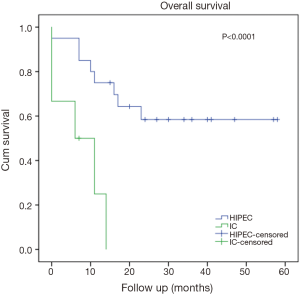

Full table
Table 5 list the distribution of disease and palliative procedures performed for the two most common pathologies (colorectal N=11, appendix N=6) who underwent aborted cytoreduction. The decision to abort cytoreduction and not proceed with CRS/HIPEC was made with a second surgeon. The reasons for aborted CRS included significant disease burden precluding a CCR score 0/1 resection (N=21) and hemodynamic instability (N=2). The PCI score for those undergoing aborted CRS was 32.2±6.4 for appendix pathology and 21.2±10.0 for colorectal pathology. Involvement of more than 9 regions, the porta hepatis, lesser omentum and widespread small intestine serosa/mesentery involvement were common factors associated with aborted cytoreduction. Palliative HIPEC (in the absence of extensive cytoreduction) was performed for two patients with refractory ascites who had required multiple paracentesis procedures prior to consideration for CRS/HIPEC.
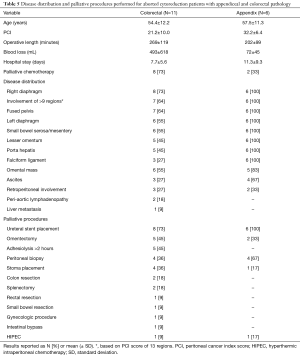
Full table
Discussion
As we reviewed our early institutional experience managing peritoneal surface malignancies, we identified that almost one-third of patients were found to have advanced disease precluding complete cytoreduction. We identified that the burden of disease and a colorectal primary tumor conferred a negative impact in this population. Less than half of the patients who underwent aborted CRS received palliative chemotherapy. The outcome for this population was poor with approximately 80% of aborted cytoreduction patients eventually succumbing to progressive disease.
Other institutions have reported similar outcomes for those who undergo aborted CRS procedures. Authors from the Netherlands reported that up to 25% of colorectal patients with PC underwent “open and close” procedures (10). The primary reason for aborted CRS was widespread disease and a preoperative stoma and an ASA score of 3 were associated with increased risk of open and close procedures. Given the poor prognosis for those undergoing aborted procedures, one would expect patients to suffer a rapidly progressive clinical course. Rodt and colleagues noted, however, that a nontherapeutic laparotomy did not negatively impact the clinical course of those found to have advanced disease precluding successful completion of CRS/HIPEC (14). In their series, the median survival was 12.7 months for colorectal cancer patients and 88% of patients received palliative chemotherapy. We observed that only about half of the patients who underwent aborted CRS procedures went on to receive palliative chemotherapy and the outcome was worse—6 months for appendiceal adenocarcinoma pathology and 8 months for colorectal pathology. The Dutch similarly observed that patients with PC who underwent aborted CRS procedures fared worse (10). They noted that approximately 50% were found to have widespread peritoneal disease precluding successful CRS/HIPEC. The median survival was 11.2 months for patients treated with palliative chemotherapy compared to 2.7 months with palliative care alone.
As a newly established peritoneal surface malignancy center, an aggressive attempt to resect all visible disease was made as evidenced by the high proportion of patients who underwent ureteral catheter placement, the length of surgery and associated procedures performed before the decision to abort cytoreduction. Similar to more established centers, at the time of exploration we calculated the burden of disease (PCI) after adhesiolysis and then began to resect disease, focusing efforts on the pelvis or diaphragm as these areas are most difficult to clear (15). Despite our sincere efforts, many of these patients were ultimately deemed unresectable due to significant disease burden and underwent aborted cytoreduction. For these patients, the decision to abort cytoreduction was made with a second surgeon. Our inexperience and judgement may have negatively affected outcome, offering CRS/HIPEC to some patients who may have been deemed to be unresectable by more experienced centers. Polanco and colleagues noted that 180 procedures are required to maximize operative outcomes and achieve the lowest risk of incomplete cytoreduction (16). The high incidence of aborted CRS procedures in our first 74 CRS/HIPEC attempts highlights the importance of the cytoreduction “learning curve” and also resulted in a re-evaluation of our preoperative assessment of patients with PC.
As demonstrated above, the accurate preoperative determination of patients who are ideal candidates for CRS/HIPEC remains a challenge. Cross sectional imaging has limited sensitivity in detecting peritoneal metastases, often underrepresenting the actual burden of disease (17). Selective use of diagnostic laparoscopy as a screening method may more accurately predict optimal candidates for CRS/HIPEC. Since review of this early experience of aborted CRS procedures, we have begun to perform diagnostic laparoscopy more liberally as a screening method for high-grade appendiceal and colorectal pathologies. Diagnostic laparoscopy is safe with low morbidity, even in the setting of prior operation and allows complete peritoneal assessment in most patients (18). The use of diagnostic laparoscopy earlier in our experience may have prevented several of the aborted cytoreduction procedures. Diffusion-weighted MRI is another method to screen potential CRS/HIPEC candidates as preoperative MRI correlates well with surgical PCI and postoperative resection status (19,20).
Disease burden, as determined by the PCI score, has been validated as a useful surrogate for successful completion of CRS/HIPEC and has a significant impact on outcome (21). We identified PCI score to be an independent predictor of OS for patients who undergo aborted cytoreduction and an important determinant of likelihood of successful completion of CRS/HIPEC. Consistent with others, we consider a PCI score of 20 as a general cut off for proceeding with CRS/HIPEC (22). We identified that the median PCI score was 10 points higher for those who underwent aborted cytoreduction procedures. Some have reported that long-term survival is possible for patients with a high PCI if able to achieve complete cytoreduction (23). We did not observe this trend, however, as advanced disease precluding a CCR 0/1 resection was the primary indication for aborted cytoreduction in 21 patients (91%). Two patients were aborted because of hemodynamic instability, one of whom underwent eventual successful CRS/HIPEC 6 months later and the other who developed recurrent disease and remains on palliative chemotherapy.
Primary tumor pathology has a significant effect on overall outcome and successful completion of CRS/HIPEC. The outcome for appendiceal primary tumors is generally more favorable than for PC from other pathologies (21). We identified a survival advantage for appendiceal primaries who were successfully able to undergo CRS/HIPEC (Figure 2). Colorectal primary tumors were the second most common pathology treated accounting for 25% of patients in this series. There were a disproportionately higher percentage of patients with colorectal pathology who underwent aborted procedures, which was identified as an independent predictor of worse outcome (Table 4, Figure 3). Several factors have been associated with increased risk of aborted CRS for colorectal peritoneal metastases including extensive disease at the porta hepatis (24), PCI score (25), extensive small bowel involvement (7), liver metastasis as well as involvement of the lesser sac and diaphragm (26). Indeed, several of these factors were present for both appendiceal and colorectal primaries (Table 5). Of note, palliative HIPEC was performed in two patients with refractory malignant ascites as HIPEC is effective in controlling ascites even when complete cytoreduction is not feasible (27).
There are several limitations to this early experience. As previously described, the liberal use of screening laparoscopy may have avoided an unnecessary laparotomy in many of the patients described in this series. As a newer peritoneal surface malignancy program, our volume, while steadily increasing, is less than more established centers and these data presented highlight some of our institutional learning curve experiences. Nonetheless, as our experience has grown, so also has our awareness of proper patient selection, clinical judgement and technical proficiency. Kusamura and colleagues noted that approximately 80–100 cases were necessary to achieve short-term prognostic gains with approximately 140–150 cases necessary to gain competence in CRS and HIPEC (28). Lastly, many patients were referred from both community and academic oncology practices. Upon review of records, it was not always clear why some patients did or did not receive neoadjuvant or adjuvant/palliative therapy as part of their treatment. This underscores the significance of a multidisciplinary treatment approach for the management of PC and standardization of treatment recommendations.
Conclusions
Complete cytoreduction and HIPEC offers the possibility for cure for appropriately selected patients with PC. The burden of disease and CCR has a profound impact on survival with less disease and complete cytoreduction conferring the best outcome. Colorectal adenocarcinoma patients who undergo aborted cytoreduction procedures have the worst outcome. While approximately half of the patients who undergo aborted cytoreduction procedures are treated with palliative chemotherapy, the outcome remains poor and underscores the importance of proper patient selection.
Acknowledgements
None.
Footnote
Conflicts of Interest: This work was presented in part at the 11th Annual Regional Therapies Meeting, February 13–15, 2016, Phoenix, AZ, and the American College of Surgeons Clinical Congress, October 17–20, 2016, Washington, DC, USA.
Ethical Statement: Approval for this study was obtained from the University of Tennessee Health Science Center (17-05132-XP) and St Jude Children’s Research Hospital Institutional Review Boards (XPD17-069). Informed consent was not required as this was a retrospective study.
References
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426-32. [Crossref] [PubMed]
- Sugarbaker PH. New standard of care for appendiceal epithelial neoplasms and pseudomyxoma peritonei syndrome? Lancet Oncol 2006;7:69-76. [Crossref] [PubMed]
- Elias DM, Ouellet JF. Intraperitoneal chemohyperthermia: rationale, technique, indications, and results. Surg Oncol Clin N Am 2001;10:915-33. xi. [PubMed]
- Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol 2005;2:3. [Crossref] [PubMed]
- Van Sweringen HL, Hanseman DJ, Ahmad SA, et al. Predictors of survival in patients with high-grade peritoneal metastases undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Surgery 2012;152:617-24; discussion 624-5. [Crossref] [PubMed]
- Mirnezami R, Mehta AM, Chandrakumaran K, et al. Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br J Cancer 2014;111:1500-8. [Crossref] [PubMed]
- Winer J, Zenati M, Ramalingam L, et al. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2014;21:1456-62. [Crossref] [PubMed]
- Esquivel J, Chua TC, Stojadinovic A, et al. Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol 2010;102:565-70. [Crossref] [PubMed]
- Pomel C, Appleyard TL, Gouy S, et al. The role of laparoscopy to evaluate candidates for complete cytoreduction of peritoneal carcinomatosis and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 2005;31:540-3. [Crossref] [PubMed]
- van Oudheusden TR, Braam HJ, Luyer MD, et al. Peritoneal cancer patients not suitable for cytoreductive surgery and HIPEC during explorative surgery: risk factors, treatment options, and prognosis. Ann Surg Oncol 2015;22:1236-42. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [Crossref] [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Rodt AP, Svarrer RO, Iversen LH. Clinical course for patients with peritoneal carcinomatosis excluded from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2013;11:232. [Crossref] [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am 2003;12:703-27. xiii. [Crossref] [PubMed]
- Polanco PM, Ding Y, Knox JM, et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann Surg Oncol 2015;22:1673-9. [Crossref] [PubMed]
- de Bree E, Koops W, Kroger R, et al. Peritoneal carcinomatosis from colorectal or appendiceal origin: correlation of preoperative CT with intraoperative findings and evaluation of interobserver agreement. J Surg Oncol 2004;86:64-73. [Crossref] [PubMed]
- Marmor RA, Kelly KJ, Lowy AM, et al. Laparoscopy is Safe and Accurate to Evaluate Peritoneal Surface Metastasis Prior to Cytoreductive Surgery. Ann Surg Oncol 2016;23:1461-7. [Crossref] [PubMed]
- Klumpp B, Aschoff P, Schwenzer N, et al. Correlation of preoperative magnetic resonance imaging of peritoneal carcinomatosis and clinical outcome after peritonectomy and HIPEC after 3 years of follow-up: preliminary results. Cancer Imaging 2013;13:540-7. [Crossref] [PubMed]
- Low RN, Barone RM, Lucero J. Comparison of MRI and CT for predicting the Peritoneal Cancer Index (PCI) preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 2015;22:1708-15. [Crossref] [PubMed]
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 2010;116:5608-18. [Crossref] [PubMed]
- Quenet F, Goere D, Mehta SS, et al. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg 2011;254:294-301. [Crossref] [PubMed]
- Cashin PH, Dranichnikov F, Mahteme H. Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy treatment of colorectal peritoneal metastases: cohort analysis of high volume disease and cure rate. J Surg Oncol 2014;110:203-6. [Crossref] [PubMed]
- Cavaliere F, De Simone M, Virzi S, et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur J Surg Oncol 2011;37:148-54. [Crossref] [PubMed]
- Pestieau SR, Sugarbaker PH. Treatment of primary colon cancer with peritoneal carcinomatosis: comparison of concomitant vs. delayed management. Dis Colon Rectum 2000;43:1341-6; discussion 1347-8. [Crossref] [PubMed]
- Chua TC, Baker B, Yan TD, et al. Palliative effects of an incomplete cytoreduction combined with perioperative intraperitoneal chemotherapy. Am J Clin Oncol 2010;33:568-71. [Crossref] [PubMed]
- Randle RW, Swett KR, Swords DS, et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol 2014;21:1474-9. [Crossref] [PubMed]
- Kusamura S, Baratti D, Hutanu I, et al. The importance of the learning curve and surveillance of surgical performance in peritoneal surface malignancy programs. Surg Oncol Clin N Am 2012;21:559-76. [Crossref] [PubMed]


