Clinicopathologic features and outcomes of gastrointestinal stromal tumors arising from the esophagus and gastroesophageal junction
Introduction
Gastrointestinal stromal tumors (GISTs), rare sarcomas thought to derive from the interstitial cells of Cajal, can occur anywhere within the gastrointestinal tract. GISTs of esophageal and gastroesophageal junction (GEJ) origin are exceedingly rare, constituting less than 1% of all GIST cases with an estimated incidence of 0.1 to 0.3 per million people (1-3). As such, little is known about their clinicopathological features and clinical outcomes. Previous studies have attempted to analyze the unique features and prognostic outcomes of esophageal GISTs but have involved only a small number of cases (4-6). Much of the published work on GISTs instead focuses on the features of gastric and proximal small intestinal GISTs, as these are by far the most common primary locations (1-3). Often the GIST may be asymptomatic and found incidentally, especially in those patients undergoing endoscopies for esophageal or GEJ adenocarcinomas, but they can also cause significant morbidity and mortality (7). The aim of this retrospective analysis was to further characterize the clinicopathological features and outcomes of GISTs arising from the esophagus and GEJ.
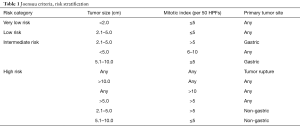
The clinical behavior of GISTs varies by location, tumor size, and mitotic activity. In 2001, Fletcher et al. in collaboration with investigators from the NIH proposed a scheme for stratifying risk of recurrent GIST following surgical resection (8). Since its publication, this classification has been widely used and supported by several large population-based studies. Subsequent modifications have been made, most notably by the Armed Forces Institute of Pathology (AFIP) which includes anatomic location (esophageal tumors are classified under the same criteria as jejunum and ileum), and the Joensuu risk criteria which simplifies classification of GIST by tumor size, location (gastric or non-gastric), and mitotic activity [<5/50, 5–10/50, and >10/50 high-powered field (HPF)] (8-11) (Table 1). Throughout the gastrointestinal tract, the risk of metastatic GIST has been shown to be strongly dependent on both tumor size and mitotic activity, with metastatic disease displaying a predilection for the liver and peritoneum (4-6,12-15). Owing to their rarity, however, esophageal and GEJ GISTs are not specifically addressed by the NIH, AFIP, or Joensuu criteria (8-11).
Full table
The discovery of KIT mutations as genetic drivers of GISTs resolved longstanding confusion concerning the diagnosis of GIST and provided a very sensitive diagnostic biomarker (16). Eighty-five percent of GISTs are KIT mutant and this corresponds to overexpression of KIT, detectable by immunohistochemistry. Approximately 15% of GISTs have mutations in platelet-derived growth factor receptor alpha (PDGFRA), BRAF, SDHB, or NF-1, but the majority of these cases still show KIT overexpression by immunohistochemistry (17). The association of KIT and PDGFR mutations with prognosis is complicated and patients with identical mutations have widely varying risks of recurrence (18). Histologically, the cells of GIST can be classified as spindle shaped, epithelioid, or mixed (19-21).
The mainstay of treatment for localized esophageal or GEJ GIST is surgery, either enucleation or esophagectomy (22), however, just over half will remain recurrence-free at 5 years (23,24). Adjuvant treatment with imatinib mesylate, an inhibitor of multiple tyrosine kinases including KIT, can reduce the recurrence rate following surgical resection (25,26), with some evidence that longer duration of treatment improves recurrence-free and overall survivals (OS) further (27-30). Neoadjuvant imatinib mesylate can be considered in select cases, especially for patients with larger tumors or those having tumors in locations that would make complete resection challenging (31-34). For metastatic or recurrent GIST, imatinib mesylate is the mainstay of therapy, with resection being uncommon (35,36). There remains considerable uncertainty regarding the optimal management of very small GISTs (<2 cm in size) in any anatomical location (27-30,37). Serial observation may be an option for carefully selected patients with very small GISTs (38).
In this retrospective analysis, we sought to further characterize the clinicopathological features and outcomes of GISTs arising from the esophagus and GEJ, with comparisons made to GISTs arising from other sites.
Methods
Patient selection
A retrospective analysis was performed on all cases of esophageal and GEJ GIST evaluated at each of the three Mayo Clinic sites (Minnesota, Florida, and Arizona) between 1997 and 2016. Inclusion criteria included adults over age 18 with a diagnosis of GIST arising in the esophagus or GEJ, based on well-established criteria (39-42). Those with a second concurrent malignancy were excluded from survival analyses. All available slides were re-reviewed by two pathologists (RPG and ALF). In order to provide a more comprehensive descriptive overview, we also queried the National Cancer Database (NCDB) and found an additional 1,010 cases between 2004 and 2014. The NCDB is sourced from hospital registry data from more than 1,500 facilities in the United States, and captures an estimated 70% of all newly diagnosed malignancies, making it an especially useful tool in studying rare malignancies (43). Only patients with confirmed histologic diagnosis of GIST were included among the NCDB data. Patients with more than two malignancies (non-melanoma skin cancers are reported to the NCDB), patients not treated at the reporting facility, and patients without completed follow-up were excluded. Those with GIST diagnosed in 2013 and 2014 were excluded from survival analysis.
Clinical and pathological features
Available clinical records were analyzed data for patient age and gender, the presence of symptoms (dysphagia, pain, bleeding, reflux), the time from symptom onset to diagnosis, the tumor location (upper third of esophagus, middle third of esophagus, lower third of esophagus, GEJ/cardia), the time to surgery from diagnosis, the type of surgery (enucleation or esophagectomy), the presence or absence of metastases, and any adjuvant therapies. The patients were staged according to the 7th edition of the AJCC manual. The time to recurrence or distant metastases after surgery and survival time from diagnosis were noted. Vital status for patients in this study was assessed until September 15, 2016. Two patients had a second concurrent malignancy and were excluded from survival analyses.
Evaluated pathological parameters included tumor size, tumor morphology (spindled, epithelioid, or mixed), mitotic index (<5/50, 5–10/50, >10/50 HPF), immunohistochemical findings (CD117/KIT, CD34, DOG-1, desmin, smooth muscle actin, S-100 protein), and molecular diagnostics (KIT exon 9 mutation, KIT exon 11 mutation, PDGFRA exon 12 mutation, PDGFRA exon 18 mutation).
Statistical methods
All statistical analyses were performed using JMP and SAS software (SAS Institute, Cary, NC, USA). The Mayo Clinic Center for Clinical and Translational Science (CCaTS) consultative services were used for biostatistics support. Continuous features were summarized with means, medians, and ranges. Categorical features were summarized with frequency and percentages. Fisher’s exact test was used to examine associations between two nominal variables. OS was estimated using the Kaplan-Meier method. Univariate associations of gender and location with OS (time from diagnosis to death) were evaluated using Cox proportional hazards regression models and summarized with hazard ratios and 95% confidence intervals. A multivariable Cox proportional hazards regression model was also established considering the variables of gender, stage (I–IV), and location (cardia or non-cardia). P values <0.05 were considered significant.
Results
Clinical features
In total, 28 cases of esophageal and GEJ GISTs evaluated at Mayo Clinic were identified and included. An additional 1,010 cases from the NCDB were analyzed. GISTs were more commonly seen in men (59.2% of Mayo Clinic cases, 52.3% of NCDB cases), and median age at diagnosis was 69 years (range, 43–89 years) in the Mayo Clinic cohort and 66 years (range, 21–90 years) in the NCDB cohort. Among the patients seen at Mayo Clinic, dysphagia (23%), pain (19%), reflux (15%), and bleeding (8%) were the most common symptoms at presentation; 23% of patients were asymptomatic at the time of diagnosis.
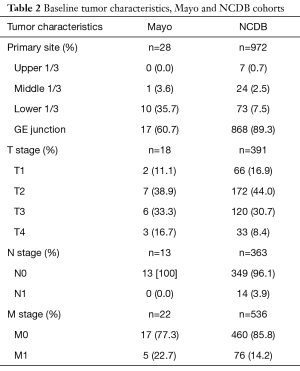
The majority of tumors in both cohorts were found at the GE junction (60.7% at Mayo Clinic, 89.3% in NCDB). In our cohort, 11 cases (39.3%) originated in the lower third of the esophagus, with 7.5% of the NCDB cohort. Among both cohorts, only about 3% of cases arose from the more proximal esophagus.
AJCC T staging was recorded in 18 of the 28 Mayo Clinic cases (T1 11.1%, T2 38.9%, T3 33.3%, T4 16.7%). No patient had nodal involvement at diagnosis. Five of 22 (22.7%) patients presented with metastases, all to the liver. Among the NCDB cohort, 16.9% (66 of 391) were T1 stage, 44% [172] T2 stage, 30.7% [120] T3 stage, and 8.4% [33] T4 stage. Only 3.9% of NCDB cases (14/363) had nodal involvement, with 14.2% (76/536) having distant metastases (Table 2).
Full table
Pathological features
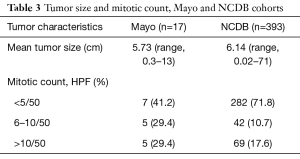
The mean tumor size in the Mayo Clinic cohort was 5.73 cm (range, 0.3–13 cm) and 6.14 cm (range, 0.02–71 cm) in the NCDB cohort. Mitotic activity was <5 per 50 HPF in 7 of 17 cases (41.2%), 6–10 per 50 HPF in 5 cases (29.4%), and >10 per 50 HPF in 5 cases (29.4%) at Mayo Clinic. Of 393 NCDB cases with available information on the mitotic count, 282 (71.8%) had <5 mitoses per 50 HPF, 42 (10.7%) had 6–10 mitoses per 50 HPF, and 69 (17.6%) had >10 mitoses per 50 HPF (Table 3).
Full table
Molecular analysis was performed on ten Mayo Clinic cases, with KIT exon 11 mutations found in eight (80%). Two cases were regarded as wild-type, with testing for mutations in PDGFRA exons 12, 14, and 18 and c-kit exons 8, 9, 11, 13, and 17 performed on both. One of the wild-type cases was found to be BRAF V600E positive with retained SDH expression via IHC. The majority of NCDB cases did not have mutational information available. Of those with information available, mutations were predominantly in KIT exon 11 (25/61, 41%), followed by KIT exon 17 (14/61, 23%), KIT exon 9 (3/61, 4.9%), and KIT exon 13 (2/61, 3.3%). KIT mutations were not identified in 17 (27.9%) of the NCDB cases.
At Mayo Clinic, tumor morphology was spindled in 12 of 15 cases (80%), epithelioid in 1 case (6.7%), and mixed in 2 cases (13.3%). By immunohistochemistry, the tumors were positive for CD117 (KIT) (23/23 cases, 100%), DOG1 (6/6, 100%), CD34 (12/14, 85.7%), smooth muscle actin (5/18, 27.8%), desmin (2/11, 18.2%), and S-100 protein (2/15, 13.3%).
OS and recurrences
Mayo Clinic cohort
The median time to diagnosis from symptom onset was 2 months (range, 0–29 months), whereas the median time to surgery from diagnosis was 1 month (range, 0–9 months). Surgery was performed in 17 of 26 (65.4%) patients, with the majority of those undergoing esophagectomy (12/17, 70.6%) compared to enucleation (5/17, 29.4%). In all, 14 of 22 patients (63.6%) patients received adjuvant therapy, all with imatinib mesylate.
Overall, eight patients did not receive adjuvant imatinib mesylate, while nine did not undergo surgery. Four patients received neither surgical resection nor adjuvant imatinib mesylate. One patient had a tumor 3.5 cm in size with unknown mitotic activity, and has had stable disease via serial imaging for over 10 years. Of the other three cases, all of the tumors were 2 cm or less in size with no mitotic activity. One remained stable on serial CT imaging for 3 years before the patient died of another cause. The second case has remained stable for over 10 years. The third patient is currently undergoing serial CT imaging with stable disease over the past 18 months.
Clinical follow-up was available for 10 patients (median 31.5 months; range, 10–145 months). In all, 7 of 18 cases (38.9%) had metastatic or recurrent disease. Of these, five presented with liver metastases. Of the patients with localized disease at the time of diagnosis, one had a local recurrence and one suffered a liver metastasis. The first patient (male) underwent esophagectomy with adjuvant imatinib for T3N0M0 high-risk (9 cm, 6–10 mitoses/50 HPF) disease and developed recurrence at the anastomotic site approximately 24 months after surgery. He subsequently resumed imatinib and received 10 fractions of localized radiation with stable disease as of most recent follow-up. The second patient (female) underwent laparoscopic enucleation without adjuvant therapy for T2N0M0 high-risk disease (4.5 cm, 10 mitoses/50 HPF) and developed metastases to the liver just over 50 months after surgery. She was subsequently initiated on imatinib, with radiographic partial response prior to being lost to follow-up.
The median tumor size and mitotic rate for those esophageal and GEJ GISTs which either presented with metastases or subsequently metastasized was 7.5 cm (range, 3.2–10.5 cm) and 90/50 HPF (range, 7–500), respectively. In contrast, median tumor size and mitotic rate for those patients without evidence of metastasis was 4 cm (range, 0.3–10.5 cm) and 6/50 HPF (range, 0–100). There was a significant association seen among metastatic disease and mitotic count >5/50 HPF (P=0.016), but no association between tumor size and metastatic disease. Of the two metastasizing tumors on which mutational analysis was performed, both had KIT exon 11 mutations. For non-metastasizing tumors, 5 of 7 cases (71.4%) had KIT exon 11 mutations, with no mutation found on the remaining 2 cases (28.6%).
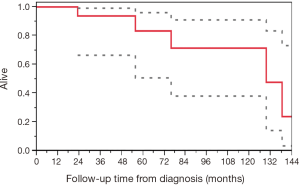
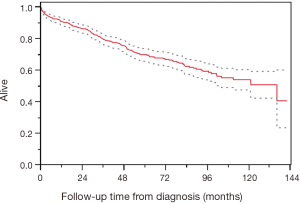
The median survival from time of surgery was 124.3 months (95% CI, 23.0–137.9) and the median OS from diagnosis was 129.5 months (95% CI, 55.7–not reached) (Figure 1).
NCDB cohort
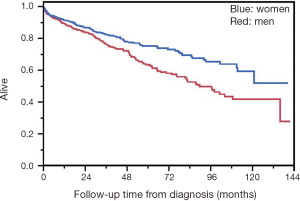
Median OS in the NCDB cohort was 135.9 months (95% CI, 104.08–not reached) (Figure 2). Women had superior survival compared to men (HR 0.67, 95% CI, 0.49–0.89, P=0.006) (Figure 3). There was no difference in rate of metastatic disease when genders were compared. Among the entire cohort of esophageal and GEJ GISTs, there was no significant difference in OS when comparing tumor location, tumor size, mitotic count, or type of surgical resection. For metastatic disease, median tumor size was 7.3 cm (range, 1–66 cm) compared to 4.8 cm (range, 0.02–71 cm) for non-metastatic disease, which was statistically significant (P≤0.0001).
Two hundred and fifty-eight of the NCDB cases were risk stratified using the Joensuu criteria (11). Thirty-one of these were very low risk, of which none were metastatic. Among 89 low risk category tumors, only 2 (2.2%) were ultimately metastatic. A total of 10.9% (15/138) of high risk category tumors were metastatic. Tumor rupture was not reported to the NCDB. As all tumors were non-gastric, none fell under the intermediate risk category.
Discussion
In this, the largest single institution study of esophageal and GEJ GISTs to date, we have found such tumors to most often localize to the GE junction and distal esophagus. This is consistent with previously published data and likely reflects the normal microanatomical distribution of interstitial cells of Cajal within the esophagus (4,5,44). The average tumor size of our cohort at Mayo (5.4 cm) was consistent with prior findings in esophageal GIST and those at other locations (4-6,9,10). Not surprisingly, the majority of our cases had mutated KIT, all in exon 11, but there were 2 presumed wild types. It has been previously reported that esophageal GIST are more likely to have wild-type status than gastric GIST (5). Rates of KIT mutations outside of exon 11 were higher than expected in the NCDB cohort (23% KIT exon 17, 4.9% KIT exon 9, 3.3% KIT exon 13), likely due to secondary mutations that developed after imatinib therapy, although it is difficult to draw firm conclusions about the prevalence of individual mutations given the small minority of cases that had mutational analysis available.
Given their rarity, esophageal GISTs are not specifically addressed by any of the prevailing GIST risk stratification schemes, including the NIH, AFIP, and Joensuu criteria (8-11,14). However, among the cases at Mayo Clinic, just under half of the tumors that would fit into the high risk category had metastases or recurrence. There were no tumors that would have fit into the intermediate risk category. Of the tumors that would be considered very low or low risk, none had recurrence or metastasis. The NCDB data showed a similar trend, with 88% of all metastatic tumors falling into the high risk category, demonstrating that these risk stratification schemes do have some utility in esophageal and GEJ GISTs.
We found a statistically significant association between metastatic disease and mitotic count >5/50 HPF in the Mayo Clinic cohort, and between metastatic disease and tumor size in the NCDB cohort. Of the four patients at Mayo Clinic with low-risk disease who received neither surgical resection nor adjuvant imatinib mesylate, all had good outcomes, offering some evidence that active surveillance might have more of a role in low risk disease with small primary tumor size and minimal mitotic activity in carefully selected patients.
The 39% rate of recurrence or metastases seen in the Mayo Clinic cohort is higher than has been seen in previously published studies, which showed risks of recurrent or metastatic disease in esophageal GIST ranging from 22.6% to 25.5% (4,5). This may reflect referral bias, as a significant percentage of Mayo Clinic patients had metastatic disease at presentation. In comparison, the rate of metastatic disease in GISTs at other sites ranges from 9% (rectum) to 26% (small intestine) (3). Previous studies have noted esophageal GIST to have inferior survival, larger tumor size, and higher mitotic rates when compared to gastric GIST, and it has been hypothesized that the lack of a serosal covering in the esophagus gives these tumors a greater propensity to metastasize (3-5).
We found a median OS in the Mayo Clinic cohort of 129.5 months with 5-year OS 85.7%, with median OS of 135.9 months and 5-year OS of 68.2% in the NCDB cohort. This compares to previously reported 5-year OS of 48.3–65.1% in esophageal GISTs (4,5). The relatively limited duration of follow-up likely explains the higher than expected OS we found, as we know even metastatic tumors can be imatinib responsive for years. Differences in anatomical location within the esophagus and GEJ are unlikely to explain any survival difference given the similarities in outcomes we found between the GISTs in the different locations. We cannot exclude that differences in therapy, both medical and surgical, with frequent use of adjuvant imatinib mesylate, affected the survival in these patient cohorts. We know that the patients from Mayo Clinic had a short time to diagnosis (2 months from symptom onset) and surgery (1 month from diagnosis), which could theoretically explain some survival benefit, although further research would be necessary to more fully investigate this difference.
Our results also showed that females had superior OS when compared to males, which has been previously observed in GISTs of the stomach and small intestine (14,45). The explanation behind this is not clear as there was no significant difference between risk of metastatic disease and gender.
Limitations
Although this is the largest single-institution cohort published to date, given the rarity of GISTs, especially those of the esophagus, we were only able to identify and analyze a limited number of cases, which in some cases had very limited data and follow-up. Many patients came from outside institutions and were seen only once at Mayo for confirmation of diagnosis, and were then subsequently treated at a different institution. Our institution had a higher rate of recurrent or metastatic disease than has been previously published in the literature for esophageal GIST, and it is very possible we missed additional cases of recurrence due to inadequate follow-up (4,5). It is also plausible, however, that the rate of recurrent or metastatic disease seen at Mayo Clinic is overestimated given the low sample size, as patients with non-metastatic disease might have been less likely to return to Mayo Clinic for follow-up. The superior survival in the Mayo Clinic cohort may in part be secondary to referral and selection biases with healthier patients being more likely to be seen at a large-volume tertiary center. This phenomenon has also been observed for other malignancies where outcomes in single-center studies are superior to the outcomes seen in population-based registry studies (46). Direct comparisons between the Mayo Clinic and NCDB cohorts were unable to be made given likely crossover between the two cohorts.
It has been well demonstrated that tumor size and mitotic activity are significant prognostic factors for GISTs throughout the GI tract (4,5,22-27). Previous studies have also shown worse outcomes in esophageal GISTs compared to gastric GISTs, with larger primary tumor sizes and thus higher proportions of high-risk classifications (4,5). Unfortunately, with the limited number of cases and subsequent deaths in our institutional cohort, a more exhaustive survival analysis was not statistically possible.
The NCDB lists all tumors with mitotic count >11/50 HPF under the same code, so we were unable to further analyze the association between mitotic count and metastatic disease with this cohort. There is no centralized pathology review within the NCDB, relying instead on the individual institutions’ reporting. Limited treatment data is available, as the database captures only whether the chemotherapy was single-agent or multi-agent, but does not identify the specific agent itself. Since cases are reported to the NCDB only from Commission on Cancer facilities, a proportion of cancers are missed, especially those more often managed in a physician’s office.
Given that a number of tumors in the NCDB cohort presented in young patients with lymph node metastases, it is possible some of the included cases were actually advanced SDH-deficient gastric GISTs, although this information is not captured in the data submitted to the NCDB by individual institutions. Furthermore, it is difficult to draw any firm conclusions about the prevalence of individual mutations, as only a small minority of patients in the NCDB cohort had any mutation testing available.
Conclusions
Overall, this project confirmed that GISTs of the esophagus and GEJ have similar primary tumor sizes, higher risk of recurrent or metastatic disease, and inferior survival to GISTs of other locations (3-5). Recurrences after surgical resection do occur, even with adjuvant therapy. Metastatic disease was associated with increased mitotic count and increased tumor size. Based on the higher OS seen in the Mayo Clinic cohort, early surgery and adjuvant imatinib mesylate might improve outcomes. However, this improved OS might also be secondary to selection bias of patients. Men were more likely than women to present with metastatic disease and had inferior OS. The Joensuu risk criteria were validated for risk stratification of esophageal and GEJ GISTs. Patients with low risk tumors can be observed for years with good outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: The study was approved by Mayo Clinic Institutional Review Board (No. IRB00000020) and informed consent was taken from all the patients.
References
- Kukar M, Kapil A, Papenfuss W, et al. Gastrointestinal stromal tumors (GISTs) at uncommon locations: a large population based analysis. J Surg Oncol 2015;111:696-701. [Crossref] [PubMed]
- Monges G, Bisot-Locard S, Blay JY, et al. The estimated incidence of gastrointestinal stromal tumors in France. Results of PROGIST study conducted among pathologists. Bull Cancer 2010;97:E16-22. [PubMed]
- Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 2005;100:162-8. [Crossref] [PubMed]
- Feng F, Tian Y, Liu Z, et al. Clinicopathologic Features and Clinical Outcomes of Esophageal Gastrointestinal Stromal Tumor: Evaluation of a Pooled Case Series. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Lott S, Schmieder M, Mayer B, et al. Gastrointestinal stromal tumors of the esophagus: evaluation of a pooled case series regarding clinicopathological features and clinical outcome. Am J Cancer Res 2014;5:333-43. [PubMed]
- Miettinen M, Sarlomo-Rikala M, Sobin LH, et al. Esophageal stromal tumor: a clinicopathological, immunohsitochemical, and molecular genetic study of 17 cases and comparison with esophageal leiomyomas and leiomyosarcomas. Am J Surg Pathol 2000;24:211-22. [Crossref] [PubMed]
- Abraham SC, Krasinskas AM, Hofstetter WL, et al. “Seedling” mesenchymal tumors (gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. Am J Surg Pathol 2007;31:1629-35. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Miettinen M, Makhlouf H, Sobin LH, et al. Gastrointestinal stromal tumors of the jejunum and ileum: a clinicopathologic, immunohistochemical, and molecular genetic study of 906 cases before imatinib with long-term follow-up. Am J Surg Pathol 2006;30:477-89. [Crossref] [PubMed]
- Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 2005;29:52-68. [Crossref] [PubMed]
- Joensuu H, Vehtari A, Riihimäki J, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [Crossref] [PubMed]
- Burkill GJ, Badran M, Al-Muderis O, et al. Malignant gastrointestinal stromal tumor: distribution, imaging features, and pattern of metastatic spread. Radiology 2003;226:527-32. [Crossref] [PubMed]
- Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112:608-15. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [Crossref] [PubMed]
- Rutkowski P, Nowecki ZI, Michej W, et al. Risk criteria and prognostic factors for predicting recurrences after resection of primary gastrointestinal stromal tumor. Ann Surg Oncol 2007;14:2018-27. [Crossref] [PubMed]
- Rubin BP, Heinrich MC. Genotyping and immunohistochemistry of gastrointestinal stromal tumors: an update. Semin Diagn Pathol 2015;32:392-9. [Crossref] [PubMed]
- Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 2008;53:245-66. [Crossref] [PubMed]
- Joensuu H, Rutkowski P, Nishida T, et al. KIT and PDGFRA mutations and the risk of GI stromal tumor recurrence. J Clin Oncol 2015;33:634-42. [Crossref] [PubMed]
- Singer S, Rubin BP, Lux ML, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 2002;20:3898-905. [Crossref] [PubMed]
- Wieczorek TJ, Faquin WC, Rubin BP, et al. Cytologic diagnosis of gastrointestinal stromal tumor with emphasis on the differential diagnosis with leiomyosarcoma. Cancer 2001;93:276-87. [Crossref] [PubMed]
- Winant AJ, Gollub MJ, Shia J, et al. Imaging and clinicopathologic features of esophageal gastrointestinal stromal tumors. AJR Am J Roentgenol 2014;203:306-14. [Crossref] [PubMed]
- Robb WB, Bruyere E, Amielh D, et al. Esophageal gastrointestinal stromal tumor: is tumoral enucleation a viable therapeutic option? Ann Surg 2015;261:117-24. [Crossref] [PubMed]
- Besana-Ciani I, Boni L, Dionigi G, et al. Outcome and long term results of surgical resection for gastrointestinal stromal tumors (GIST). Scand J Surg 2003;92:195-9. [Crossref] [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [Crossref] [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 2009;373:1097-104. [Crossref] [PubMed]
- DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg 2013;258:422-9. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [Crossref] [PubMed]
- Joensuu H, Eriksson M, Sundby Hall KS, et al. Adjuvant Imatinib for High-Risk GI Stromal Tumor: Analysis of a Randomized Trial. J Clin Oncol 2016;34:244-50. [Crossref] [PubMed]
- Wang D, Zhang Q, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumors: long-term follow-up results of Radiation Therapy Oncology Group 0132. Ann Surg Oncol 2012;19:1074-80. [Crossref] [PubMed]
- Blackstein ME, Blay JY, Corless C, et al. Gastrointestinal stromal tumours: consensus statement on diagnosis and treatment. Can J Gastroenterol 2006;20:157-63. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Sarcoma (Version 2 2017). Accessed March 31, 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf
- Keung EZ, Raut CP. Management of Gastrointestinal Stromal Tumors. Surg Clin North Am 2017;97:437-52. [Crossref] [PubMed]
- Gronchi A, Raut CP. The combination of surgery and imatinib in GIST: a reality for localized tumors at high risk, an open issue for metastatic ones. Ann Surg Oncol 2012;19:1051-5. [Crossref] [PubMed]
- Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013;20:2937-43. [Crossref] [PubMed]
- DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg 2007;245:347-52. [Crossref] [PubMed]
- Raut CP, Posner M, Desai J, et al. Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 2006;24:2325-31. [Crossref] [PubMed]
- Casali PG, Jost L, Reichardt P, et al. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 2009;20 Suppl 4:64-7. [PubMed]
- Sepe PS, Brugge WR. A guide for the diagnosis and management of gastrointestinal stromal cell tumors. Nat Rev Gastroenterol Hepatol 2009;6:363-71. [Crossref] [PubMed]
- Baskin Y, Kocal GC, Kucukzeybek BB, et al. PDGFRA and KIT Mutation Status and Its Association With Clinicopathological Properties, Including DOG1. Oncol Res 2016;24:41-53. [Crossref] [PubMed]
- Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014;32:1563-70. [Crossref] [PubMed]
- Corless CL, McGreevey L, Haley A, et al. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol 2002;160:1567-72. [Crossref] [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for Outcomes Research: A Review. JAMA Oncol 2017;3:1722-8. [Crossref] [PubMed]
- Radenkovic G, Ilic I, Zivanovic D, et al. C-kit-immunopositive interstitial cells of Cajal in human embryonal and fetal oesophagus. Cell Tissue Res 2010;340:427-36. [Crossref] [PubMed]
- Call J, Walentas CD, Eickhoff JC, et al. Survival of gastrointestinal stromal tumor patients in the imatinib era: life raft group observational registry. BMC Cancer 2012;12:90. [Crossref] [PubMed]
- Strosberg JR, Halfdanarson TR. Survival analyses of pancreatic neuroendocrine tumors: Contrasting institutional databases with population-based studies. J Clin Oncol 2011;29 suppl 4:abstr 186.