Clinical impact of underutilization of adjuvant therapy in node positive gastric adenocarcinoma
Introduction
In 2017, the American Cancer Society estimates that there will be 28,000 new diagnoses of gastric adenocarcinoma and 10,960 deaths from this disease in the United States (1). The clinical outcome of gastric adenocarcinoma is strongly influenced by the status of nodal metastases, and this variable is an important determinant of overall survival. Despite the heterogeneity of outcomes in studies across the globe, lymph node involvement remains an important prognostic indicator for recurrence and survival (2-7). Patients with node negative cancer historically experience 5-year overall survivals ranging from 65–85% while the presence of N2 or N3 disease substantially decreases this range to 20–35% (8-11).
The benefit of adjuvant therapy in the treatment of gastric carcinoma was first strongly supported in 2001 by the landmark Intergroup INT-0116 trial where MacDonald and colleagues showed that adjuvant chemoradiotherapy, as opposed to resection alone, improves overall survival (HR for death in surgery-only compared to chemoradiotherapy group 1.35, P=0.005) (12). The advantages of adjuvant therapy were again demonstrated in the well-designed phase 3 trial randomizing patients to peri-operative chemotherapy (MAGIC trial) where individuals randomized to the peri-operative arm had 5-year survival rates of 36.3% vs. 23.0% with surgery alone (13). The benefits of adjuvant therapy have been supported by other clinical studies worldwide (14,15).
In particular, adjuvant therapy has a proven value when planning a gastrectomy with curative intent for node positive gastric cancer. In addition to improved survival, the purported advantages of adjuvant chemotherapy and chemoradiotherapy include an increased likelihood of an R0 resection, treatment of micrometastatic disease, and locoregional control (15,16). Despite supporting data from clinical trials and recommendations by the National Comprehensive Cancer Network, we believe that there is an underutilization of adjuvant therapy in node positive gastric cancer across the United States (17). Using the National Cancer Database (NCDB), we assess the utilization of adjuvant chemotherapy and chemoradiotherapy in the treatment of node positive gastric cancer and whether the lack of adjuvant therapy is associated with poor survival.
Methods
Study population
This study was approved by the Yale Institutional Review Board. The American College of Surgeons (ACS) NCDB was reviewed to identify patients from 2006–2013 with histologically defined gastric adenocarcinoma with clinical stage T0–T4, N1–3, M0 (tumor with any depth of invasion, any regional lymph node metastasis, no distant metastasis) who underwent resection of their primary tumor. This study was affected by transition from the 6th edition to 7th edition of the AJCC gastric cancer staging system, reflected in the NCDB starting with cancers diagnosed in 2010. There was insufficient staging information to convert all patients to either the 6th or 7th edition, so clinical and pathologic T stage were instead grouped as T0–T1, T2–T4, and TX, and number of regional nodes positive as captured by the NCDB was used instead of pathologic N staging. Invasive adenocarcinoma was defined by: ICD-O-3 histology codes (8140–8147, 821, 8220–8221, 8250–8255, 8260–8263, 8310–8319, 8480–8482, 8570–8576). Patients with incomplete clinical staging or histology were excluded. In addition, due to different tumor biology and treatment paradigms, primary tumors coded as listed in the cardia or overlapping regions of the stomach that may potentially include the cardia were excluded.
Study variables
Independent variables included age, sex, race, Hispanic ethnicity, Charlson-Deyo score (comorbidities, score modified by NCDB to 0, 1, and ≥2), insurance status, median income (calculated by the NCDB based on patient’s zip code), Greater Circle Distance (distance from patient’s zip code to hospital reporting the case), facility location, facility type, year of diagnosis, tumor location, TNM clinical and pathologic T and N stage (grouped to circumvent AJCC transition from 6th to 7th edition in 2010), number of lymph nodes examined, number of lymph nodes positive (by pathology), scope of regional lymph node surgery, tumor grade, type of gastrectomy, surgical margins, surgical inpatient stay, and 30-day unplanned readmission after surgical discharge. Treatment groups were defined as (I) no chemotherapy and/or radiation therapy, (II) pre-operative chemotherapy, (III) post-operative chemotherapy, (IV) post-operative chemoradiation, (V) peri-operative (both pre- and post-operative) chemotherapy, and (VI) other adjuvant therapy, pre- and/or post-operative, chemotherapy and/or radiation therapy, that did not otherwise meet criteria, where groups 2–6 are included in the overall classification, ‘any adjuvant therapy’. Sequence of chemotherapy was determined using sequence of systemic therapy in relationship to surgery. Patients who received chemotherapy or radiation therapy >120 days before or after surgery were not included in treatment groups. Radiation therapy was only included if it was specified to area of resection (stomach and/or associated lymph nodes or abdomen, not otherwise specified).
Data analysis
Univariate analyses were performed to evaluate clinical associations. Characteristics are reported as frequencies for defined categorical variables. Comparisons were made using Chi-square test or Fisher’s exact test.
Primary outcome was overall survival and reported by adjuvant treatment type using Kaplan Meier and log-rank test. In the Cox regression proportionality assumption was checked by including a time-varying covariate, an interaction between the covariate and event time. Proportionality assumption did not hold for treatment variable. As an alternative, we conducted stratified Cox regression analysis for each treatment group separately, and used the restricted mean survival time (RMST) as the statistic to compare across treatment groups (18,19). Chi-squared test was used to conduct a global comparison of RMST across treatment groups, and Z-test was used to conduct pair-wise comparisons. Adjusted covariates included age, median income, facility location, tumor location, clinical and pathologic T stage, number of lymph nodes examined, number of lymph nodes positive, tumor grade, Charlson-Deyo score, surgical margin, year of diagnosis, and length of surgical inpatient stay. Additional adjustments for sex, race, Hispanic ethnicity, insurance, facility type, Greater Circle Distance, unplanned readmission, and lymph node surgery did not significantly change observed RMST and were not included in final stratified Cox models. Missing values in variables were coded as unknown for multivariable modeling purposes. We chose 5 and 8 years as reference time points. To nullify effects of immortal time bias, this analysis was additionally performed among patients who survived longer than 90 days post-surgically. To adjust for patients who had positive surgical margins and therefore should have received post-operative adjuvant therapy, a supplementary analysis was built using only patients with R0 resections. A P value <0.05 was considered statistically significant, and all statistical tests were 2-sided. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
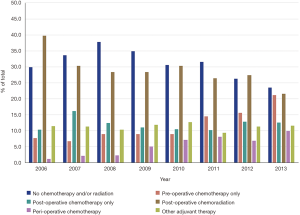
A total of 2,565 patients met inclusion criteria: 793 (30.9%) received no chemotherapy and/or radiation, 310 (12.1%) received pre-operative chemotherapy, 306 (11.9%) received post-operative chemotherapy, 723 (28.2%) received post-operative chemoradiation, 147 (5.7%) received peri-operative chemotherapy, and 286 (11.2%) received some other adjuvant therapy. Trends of the utilization of these five treatment modalities are summarized in Figure 1. Notably, peri-operative chemotherapy represented only 1.1% of the total treatment modalities in 2006, rising steadily to 9.9% in 2013. Post-operative chemoradiation has decreased from 39.7% in 2006 to 21.6% in 2013. Pre-operative chemotherapy has risen from 7.6% in 2006 to 21.1% in 2013.
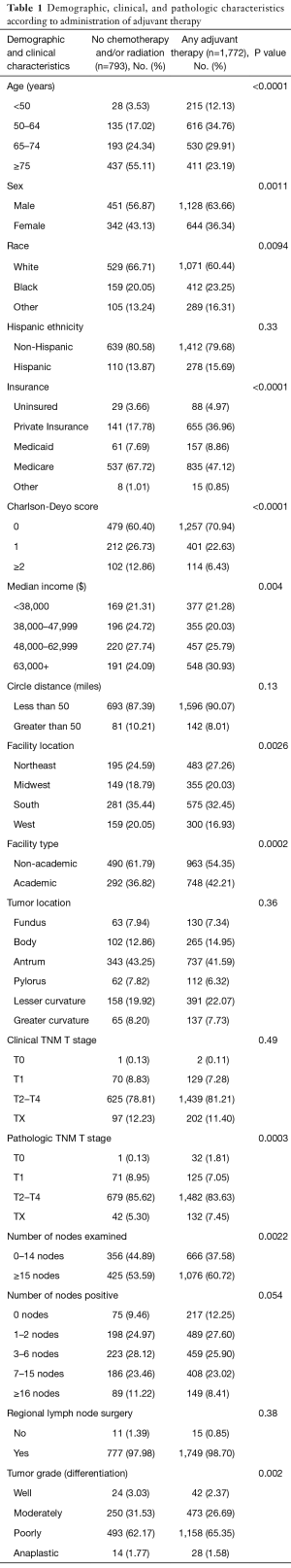
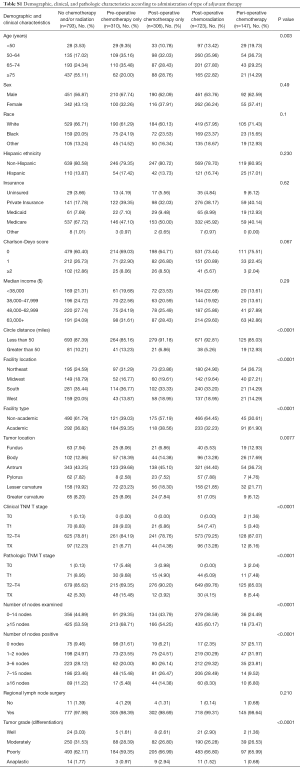
Demographic and pathologic characteristics of the study cohort are summarized in Table 1. Patients not receiving chemotherapy and/or radiation were more likely to have more comorbidities (Charlson-Deyo score ≥2). Patients undergoing any adjuvant therapy were more likely to be treated at an academic center (P=0.002). The median age of diagnosis was 77 years for patients not receiving chemotherapy and/or radiation and 66 years for patients having any adjuvant therapy (Table S1, 66 years for pre-operative chemotherapy, 67 years for post-operative chemotherapy, 65 years for post-operative chemoradiation, and 61 years for peri-operative chemotherapy). There was no association with clinical outcome in insurance and access to medical care (data not shown).
Full table
Full table
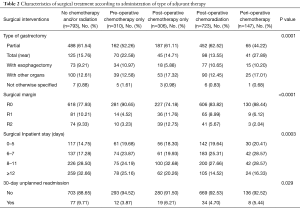
Operative characteristics are summarized in Table 2. The highest percentage of patients achieving an R0 resection were those who underwent pre-operative chemotherapy and peri-operative chemotherapy, while those least likely to undergo an R0 resection did not receive any adjuvant therapy (90.65% and 88.44% vs. 77.93%, respectively, P<0.0001). Patients who did not undergo chemotherapy and/or radiation were also more likely to have an unplanned readmission within 30 days of their primary surgery (P=0.029).
Full table
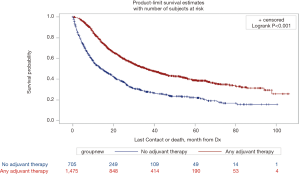
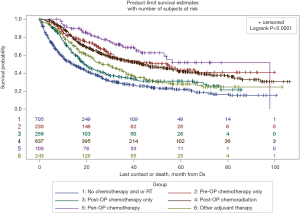
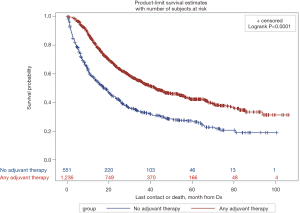
Survival data was available on 2,180 (85.0%) of patients. Mean time to follow-up was 26.9 months (SD 22.4). The median overall survival of patients who did not receive any form of adjuvant therapy was far worse than those that received any form of adjuvant therapy (13.9 vs. 36.1 months, P<0.0001, Figure 2). When examined with respect to specific form of adjuvant therapy administered, median overall survival after no chemotherapy and/or radiation was 13.9 months, after pre-operative chemotherapy was 53.6 months, after post-operative chemotherapy was 19.0 months, after post-operative chemoradiation was 43.1 months, and after peri-operative chemotherapy was 56.3 months (Figure S1, P<0.0001).
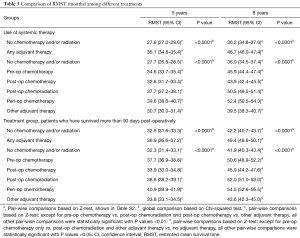
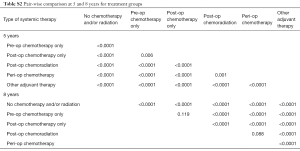
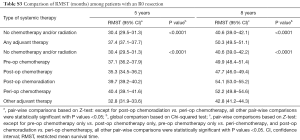
Stratified Cox regression adjusted for covariates demonstrated a significantly improved RMST of both any adjuvant therapy and individual treatment groups compared to no chemotherapy and/or radiation, over 5 and 8 years. Compared to RMSTs for 5 and 8 years of 27.7 and 36.0 months respectively for no chemotherapy and/or radiation, RMSTs for peri-operative chemotherapy were 39.6 and 52.4 months and RMSTs of post-operative chemoradiation were 37.7 and 50.5 months (Table 3, P<0.0001). Pair-wise comparisons between treatment groups demonstrated that peri-operative chemotherapy is superior to all other treatment modalities, save for post-operative chemoradiation over 8 years (Table S2, P<0.0001 and P=0.088, respectively). Similar survival benefit of adjuvant therapy was observed after exclusion of patients who did not survive 90 days post-surgically (Table 3, P<0.0001). This survival benefit of pre-operative adjuvant therapy additionally held exclusively for patients who had an R0 resection (Figure S2, Table S3, P<0.0001).
Full table
Full table
Full table
Discussion
A basic tenet in the treatment of epithelial-based gastrointestinal malignancies is the utilization of a multimodality approach, particularly for those with nodal metastases, in order to provide the best oncologic outcome. Several randomized studies of patients with gastric adenocarcinoma have convincingly demonstrated prolonged survival when surgical resection is used concomitantly with chemotherapy or chemoradiotherapy (12,13,20-22). Despite this level 1 data, we report that nearly one third of Americans with node positive gastric adenocarcinoma never receive any form of adjuvant treatment even though a multimodality approach is clearly associated with better outcomes. In 2,565 patients treated for node positive gastric cancer between 2006 and 2013, 30.9% of patients received no form of adjuvant therapy. Equally striking is that the administration of adjuvant therapy is associated with an improved RMST, P<0.0001. Clearly, there is room for improvement in the treatment of gastric cancer in the United States.
It has been over one decade since the MAGIC trial reported peri-operative chemotherapy with epirubicin-cisplatin-5-fluorouracil (ECF) improved 5-year overall survival from 23% to 36% (13). The advantages to this approach include that more patients are able to receive chemotherapy, the potential for the eradication of micrometastases, the increased probability for a margin negative resection, and the ability to “test the cancer biology” before proceeding with a major oncologic resection. Our study reinforces these benefits showing that administration of pre-operative chemotherapy is associated with R0 resection in up to 90.65% of cases versus 77.93% in cases with no neoadjuvant therapy (P<0.0001). By comparison, 10.21% and 9.33% of patients who did not receive any adjuvant therapy had an R1 and R2 resection, respectively. Despite these clear advantages and many centers choosing to use chemotherapeutic agents better tolerated than ECF (adverse effects in the MAGIC trial including cytopenias and gastrointestinal and cutaneous effects), a maximum of 9.9% of patients were treated with peri-operative chemotherapy in 2013, even seven years after the MAGIC trial’s publication. Even though 50% of patients completed pre- and post-operative chemotherapy, it is unlikely that the underutilization of the MAGIC trial regimen is strictly due to poor post-operative tolerance (17).
Gastrectomy followed by adjuvant chemoradiotherapy is also an accepted therapy for nodal positive gastric cancer, supported by level 1 data. The advantages to this tri-modality approach are sterilization of the surgical resection field and treatment of micrometastases. Mature data from the Intergroup-0116 trial was first reported over 15 years ago, and it demonstrated that patients randomized to the tri-modality arm of surgery, chemotherapy, and radiation experienced an improved median overall survival from 27 to 36 months (12,23). A subset analysis of this trial demonstrated the benefit of post-operative chemoradiotherapy in patients with node-positive gastric cancer, with a hazard ratio of 1.32 favoring overall survival (P=0.0046). Data from the more recent ARTIST trial validates this benefit, including for those with node positive disease (20). Our study substantiates the benefit to tri-modality therapy, showing that this approach provides clinical outcomes comparable to the peri-operative approach over 8 years (P>0.05) and superior to no adjuvant therapy (P<0.0001). Interestingly, our study has demonstrated more of a decline in the use of adjuvant chemoradiotherapy from 39.7% in 2006 to 21.6% in 2013. This may be reflective of the early and rapid adoption of the INT-0116 once the results were publicized. According to a SEER-Medicare study, the use of adjuvant radiation rose from 14.6% between January 1996 to April 2000 before the trial to 30.4% between May 2000 to December 2003 after the trial (P<0.001) (24).
Cancer care is becoming increasingly complex, and a prerequisite to favorable outcomes is coordinated care among practitioners from different disciplines in oncology. It is our belief that specialized oncology training enables this awareness and increases the odds for better outcomes. A systematic review of surgeon training and specialization reinforces this, finding that specialized surgeons had better outcomes than non-specialized surgeons in oncology care (25). Awareness of clinical trials and the ability to adopt one’s practice to modern evidence based medicine is crucial. There are many factors that may influence the adoption of multimodality treatment into clinical care. In our cohort, the highest percentage of use of peri-operative chemotherapy was found in academic centers, which may reflect a greater infrastructure to support coordinated care in accordance with modern evidence-based practice. Our study underscores this point where we clearly show that neglecting any major modality in the treatment of node positive gastric cancer portends worse outcomes. The clinical implication of this trend is reinforced by the marked improvement in overall survival compared to no chemotherapy and/or radiation; peri-operative chemotherapy was associated with the highest median overall survival at 56.3 months compared to other treatment regimens (P<0.0001). Interestingly, when our data is compared to historical data of similar groups of patients who undergo curative intent gastrectomy without adjuvant therapy, there are striking similarities in survival (26,27). Survival remains poor when surgery is the only modality used to treat node positive gastric adenocarcinoma.
Despite the many advantages of a large national database, this study cohort has its limitations. The NCDB is a hospital-based registry containing data from Commission on Cancer-accredited programs, and it is therefore understood that the results might not be generalizable to patients who are not treated in these hospitals. With regards to our outcome results, our assessable outcomes are limited to overall survival, as there is no information on disease progression and recurrence precluding us from commenting on disease-free survival. In clinical practice, there are likely to be more factors than are available in the NCDB that weigh on the clinical assessment as to whether or not a patient will physiologically tolerate oncologic therapy. Further, we were not able to assess treatment intent. Instead we are only able to analyze registry data for what patients actually received. It is possible that patients in our study cohort who received pre-operative chemotherapy only were also intended to receive post-operative therapy but did not complete it because of intolerance to chemotherapy, surgical complications, other illnesses, or being lost to care. It is also possible that patients did not receive post-operative chemotherapy because they had not survived long enough to receive it, which raises a concern about potential immortal time bias. Approximately 90% of the patients who underwent post-operative adjuvant therapy received it within 90 days after surgery. Therefore, we performed sensitivity analysis by excluding people who did not survive 90 days after surgery. The results showed similar survival benefits from adjuvant therapy, suggesting that immortal time bias was less likely to explain the observed survival benefits from adjuvant therapy.
Conclusions
Adjuvant chemotherapy and chemoradiotherapy after gastrectomy for node positive gastric adenocarcinoma is vastly underutilized. The importance of this phenomenon is underscored by inferior oncologic outcomes with a clinically significant decrease in median survival from 36.1 to 13.9 months for patients who are not treated with these multimodality approaches. Our findings support the administration of chemotherapy or chemoradiotherapy with gastrectomy in the treatment of node positive gastric cancer as routine standard practice because of the clear survival advantage. Future studies are needed to gain insight into this high frequency of diversion from the standard of care and potential causes that may be influencing clinical decision-making.
Acknowledgements
Funding: This publication was made possible by CTSA Grant Number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. This work was also supported by the Lampman Research Fund in Yale Surgical Oncology and the Yale University School of Medicine Medical Student Research Fellowship.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed by the Yale Institutional Review Board (No. 00000596) and deemed exempt from review as a secondary data analysis.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Kooby DA, Suriawinata A, Klimstra DS, et al. Biologic predictors of survival in node-negative gastric cancer. Ann Surg 2003;237:828-35; discussion 835-7. [Crossref] [PubMed]
- Kodera Y, Yamamura Y, Shimizu Y, et al. Lymph node status assessment for gastric carcinoma: is the number of metastatic lymph nodes really practical as a parameter for N categories in the TNM Classification? Tumor Node Metastasis. J Surg Oncol 1998;69:15-20. [Crossref] [PubMed]
- Nakamura K, Morisaki T, Sugitani A, et al. An early gastric carcinoma treatment strategy based on analysis of lymph node metastasis. Cancer 1999;85:1500-5. [Crossref] [PubMed]
- Komatsu S, Ichikawa D, Miyamae M, et al. Positive Lymph Node Ratio as an Indicator of Prognosis and Local Tumor Clearance in N3 Gastric Cancer. J Gastrointest Surg 2016;20:1565-71. [Crossref] [PubMed]
- Komatsu S, Ichikawa D, Nishimura M, et al. Evaluation of prognostic value and stage migration effect using positive lymph node ratio in gastric cancer. Eur J Surg Oncol 2017;43:203-9. [Crossref] [PubMed]
- Smyth EC, Fassan M, Cunningham D, et al. Effect of Pathologic Tumor Response and Nodal Status on Survival in the Medical Research Council Adjuvant Gastric Infusional Chemotherapy Trial. J Clin Oncol 2016;34:2721-7. [Crossref] [PubMed]
- Deng J, Liang H, Sun D, et al. Prognosis of gastric cancer patients with node-negative metastasis following curative resection: outcomes of the survival and recurrence. Can J Gastroenterol 2008;22:835-9. [Crossref] [PubMed]
- Fukuda N, Sugiyama Y, Wada J. Prognostic factors of T4 gastric cancer patients undergoing potentially curative resection. World J Gastroenterol 2011;17:1180-4. [Crossref] [PubMed]
- Dicken BJ, Bigam DL, Cass C, et al. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg 2005;241:27-39. [PubMed]
- Li B, Li Y, Wang W, et al. Incorporation of N0 Stage with Insufficient Numbers of Lymph Nodes into N1 Stage in the Seventh Edition of the TNM Classification Improves Prediction of Prognosis in Gastric Cancer: Results of a Single-Institution Study of 1258 Chinese Patients. Ann Surg Oncol 2016;23:142-8.
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210-8. [Crossref] [PubMed]
- Greenleaf EK, Hollenbeak CS, Wong J. Trends in the use and impact of neoadjuvant chemotherapy on perioperative outcomes for resected gastric cancer: Evidence from the American College of Surgeons National Cancer Database. Surgery 2016;159:1099-112. [Crossref] [PubMed]
- Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol 2013;13:152. [Crossref] [PubMed]
- Zhang X. Comparison of restricted mean survival times between treatments based on a stratified Cox model. Bio-Algorithms and Med-Systems 2013;9:183-9. [Crossref]
- Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [Crossref] [PubMed]
- Coburn NG, Guller U, Baxter NN, et al. Adjuvant therapy for resected gastric cancer--rapid, yet incomplete adoption following results of intergroup 0116 trial. Int J Radiat Oncol Biol Phys 2008;70:1073-80. [Crossref] [PubMed]
- Bilimoria KY, Phillips JD, Rock CE, et al. Effect of surgeon training, specialization, and experience on outcomes for cancer surgery: a systematic review of the literature. Ann Surg Oncol 2009;16:1799-808. [Crossref] [PubMed]
- Maehara Y, Moriguchi S, Sakaguchi Y, et al. Adjuvant chemotherapy enhances long-term survival of patients with advanced gastric cancer following curative resection. J Surg Oncol 1990;45:169-72. [Crossref] [PubMed]
- Grau JJ, Martin M, Fuster J, et al. Impact of adjuvant chemotherapy in the long-term outcome of patients with resected gastric cancer. J Surg Oncol 2003;82:234-40. [Crossref] [PubMed]