Preferential use of imaging modalities in staging newly diagnosed rectal cancer: a survey of US radiation oncologists
Introduction
Colorectal cancer is the second leading cause of cancer death in the United States (1) and the fourth most frequently diagnosed cancer, with rectal cancer representing 40–50% of cases (2). Annually, approximately 39,910 patients are newly diagnosed with rectal cancer in the United States (3). Death from colorectal cancer has decreased 35% from 1990 to 2007 due to earlier diagnoses through screening and better treatment modalities (4).
Up until 2003, endorectal ultrasound (EUS) was the only standard imaging modality recommended for staging rectal cancer (5), with sensitivity and specificity of 94% and 86%, respectively. More recently, pelvic magnetic resonance imaging (MRI) became widely used, as it has similar sensitivity and specificity to EUS (at 94% and 69%, respectively), but has an important advantage in being able to evaluate iliac lymph nodes and can accurately assess the circumferential resection margin (CRM), which is key in planning surgery and in some countries informs the choice of neoadjuvant therapy (6). CRM status by MRI evaluation is also prognostic. Based on the results of the MERCURY trial patients with negative CRM by MRI had a 5-year OS of 62.2% compared with 42.2% in patients with positive CRM (7). The current National Comprehensive Cancer Network (NCCN) guidelines recommend pelvic MRI with contrast for rectal cancer staging at diagnosis. At the same time, these guidelines discourage providers from using positron emission tomography/computed tomography (PET/CT) scans in this clinical setting. Literature investigating the sensitivity and specificity of PET is scant, however van Cutsem et al. states specificity of PET is limited due to the recognized increased intake of FDG yielding false positives (8).
The current utilization of imaging modalities for staging of newly diagnosed rectal cancer in the US by radiation oncologists is not known.
Methods
Survey instrument development and data collection
We designed an online closed survey using REDCap software licensed by the Oregon Clinical and Translational Research Institute (OCTRI). The study was approved by the institutional IRB and was tested for functionality before launch. Participants were notified the length of time to complete the survey, that it was anonymous, and the purpose of the study. The survey consisted of 14 questions pertaining to respondents’ demographics and use of imaging modalities. The online survey was sent anonymously with the internet-based REDCap data collecting software to 6,949 currently practicing potential participants. Email invitations were sent on November 16th and 17th of 2016 and a single reminder email was sent on November 30th, 2016.
Statistical analysis
Respondent characteristics (years in practice, practice setting, region of practice, number of rectal patients treated per year) were tested for association with respondents’ self-assessed utilization of imaging modalities—EUS, pelvis MRI and PET/CT—using Chi-squared test. A P value of less than 0.05 was defined as statistically significant. The reported percent of imaging utilized among all respondents using a specific modality for staging their rectal cancer patients is defined as median frequency. Staging 75% or more of rectal cancer patients with a given imaging modality was defined as high utilization.
Results
Respondent characteristics
Of the 6,949 potential participants, we received 337 failed/undelivered automatic responses, 7 non-applicable/ineligible responses and 220 completed responses. The characteristics of these 220 individuals are summarized in Table 1. Sixty one percent of respondents practice over 10 years since completion of residency program, and 61% work in private practice. Fifty five percent treat 10 or fewer patients with rectal cancer per year.
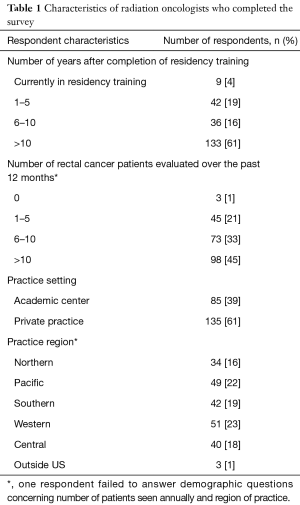
Full table
Use of imaging modalities
EUS was the most common imaging modality used by respondents, with the median utilization frequency of 75%. This was followed by PET/CT at 60% and pelvic MRI at 50% median utilization frequencies (Table 2). Figure 1 shows the overlap of high utilizers, with 9.5% of respondents classified as high utilizers of all three modalities. Thirty eight percent of respondents are high utilizers of MRI, while 52% are high utilizers of EUS (Table 2). Forty seven percent are high PET utilizers (Table 2). Of those, 27% solely use PET/CT in staging newly diagnosed rectal cancer.
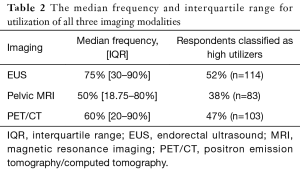
Full table
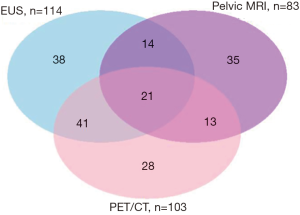
Table 3 shows association between respondent characteristics and their use of imaging modalities. Fifty two percent of respondents self-identified as high utilizers of EUS. These respondents were more likely to have 10 or more years of professional experience. In particular, 62% of those with 10 or more years of experience are high utilizers of EUS as compared with 37% of those with less than 10 years experience (P<0.0001).
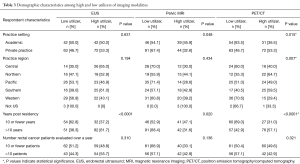
Full table
Forty seven percent of respondents were classified as high utilizers of PET/CT. Among these respondents, 31.0% of those who practiced 10 or fewer years self-identified as high utilizers, compared to 57% of those with more than 10 years’ experience (P<0.0001). Thirty seven percent of academics self-identified as high utilizers, compared with 53% of those in private practice (P=0.015).
Thirty eight percent of respondents are classified as high utilizers of pelvic MRI. Only 31.0% of those who practiced 10 or more years self-identified as high utilizers of pelvic MRI, compared to 47% of those with less than 10 years’ experience (P=0.02). Forty six percent of academics self-identified as high utilizers, compared with 33% of those in private practice (P=0.05).
Five percent of respondents report zero EUS utilization. Another 5% of respondents do not use pelvic MRI and 7% report no utilization of PET/CT during patient evaluation.
Conclusions
Accurate staging of patients with newly diagnosed rectal cancer is essential for defining the treatment strategy and counseling patients regarding the expected outcomes. EUS and pelvic MRI have similar sensitivities (94%), yet EUS has higher specificity (86%) than MRI (69%) in assessing local tumor invasion (4). However, MRI can evaluate iliac, mesenteric or retroperitoneal nodes as well as the CRM involvement. At the present time, there is no indication for PET/CT use in evaluation of patients with rectal cancer (1).
Our results show EUS continues to be the most frequent imaging modality, despite current NCCN recommendations that support MRI over EUS. One possible explanation for this discordance is the prevalent difference in experiences among treating physicians. Whereas EUS has been used in rectal cancer staging for decades, MRI is still a relatively new imaging modality. This explanation is in part supported by our observation that respondents who have been practicing for more than 10 years post-residency were more likely to rely on EUS than pelvic MRI. Of the respondents practicing more than 10 years post-residency, 61.7% were high EUS utilizers, compared with 31.6% high utilizers of pelvic MRI.
Future studies need to revisit how EUS over MRI utilization affect patient outcomes, and with time we anticipate that MRI utilization will continue to increase. This will become increasingly more important, given the emergence of new nodal contrast agents such as ultrasmall paramagnetic iron oxide (USPIO), which has been shown to increase sensitivity of detecting lymph nodes involved with malignant cells (9). As treatment personalization supplants standard one-size-fits-all guidelines, detailed information on CRM status, tumor vascularity, involvement of perirectal and lateral pelvic lymph nodes will become paramount to optimal treatment modality and sequence selection.
Of a greater concern is our finding PET/CT—the imaging modality not supported by clinical evidence—is used more frequently than pelvic MRI, especially by those further out from their residency training. PET/CT imaging is currently reimbursed by Medicare and most private insurances in the initial treatment planning and subsequent treatments for almost all solid cancers, including colorectal cancer (10). This financial incentive could be the potential culprit for inappropriate utilization of this imaging technology. Another explanation could be physicians are simply unaware of the NCCN guidelines. Comfort level of each physician could be an alternative explanation. It will be of interest to compare the rate of PET/CT utilization in other countries that do not cover PET imaging in management of rectal cancer. Further policy work and cost analysis need to shed light on the current US practice of over-utilization of PET imaging in rectal and other solid tumor malignancies, such as prostate, bladder and liver malignancies.
Finally, as evidenced from Figure 1, many clinicians order more than one imaging modality for an individual patient with a newly diagnosed rectal cancer. Whether or not ordering supplementary tests improves staging precision or treatment outcomes, and not simply escalating overall treatment costs, needs to be elucidated.
One of the greatest limitations of our study is the limited sample size of 220 completed responses. It is possible that the results were skewed by selection bias. A reimbursement-based national database analysis should be conducted to support or refute our survey findings. However, due to the delay in reporting captured clinical data, this analysis could take several years to pilot in order to determine retrospectively the true rate of imaging modalities utilization in the US in the year corresponding to the timing of our survey of late 2016.
In conclusion, there is a dramatic divide between national guidelines and the utilization of imaging modalities for initial staging of patients with rectal cancer in the United States. The most cost effective modality needs to be determined through further studies and its use enforced by policy and reimbursement changes.
Acknowledgements
We thank the respondents who have taken the time to participate and complete our survey.
Funding: This work was support by OHSU REDCap [1 UL1 RR024140 01].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board of Oregon Health and Science University (IRB protocol 11149), and the requirement to obtain informed consent from survey respondents was waived.
References
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Rectal Cancer Version 3.2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Bipat S, Glas AS, Slors FJ, et al. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology 2004;232:773-83. [Crossref] [PubMed]
- National Comprehensive Cancer Network. (2003). Clinical Practice Guidelines in Oncology-Rectal Cancer v.1.2003.
- Klessen C, Rogalla P, Taupitz M. Local staging of rectal cancer: the current role of MRI. Eur Radiol 2007;17:379-89. [Crossref] [PubMed]
- Taylor FG, Quirke P, Heald RJ, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol 2014;32:34-43. [Crossref] [PubMed]
- Van Cutsem E, Verheul HM, Flamen P, et al. Imaging in Colorectal Cancer: Progress and Challenges for the Clinicians. Cancers (Basel) 2016;8:E81. [Crossref] [PubMed]
- Kim DJ, Chung JJ, Yu JS, et al. Evaluation of lateral pelvic nodes in patients with advanced rectal cancer. AJR Am J Roentgenol 2014;202:1245-55. [Crossref] [PubMed]
- Department of Health and Human Services. Centers for Medicare & Medicaid Services. Availble online: https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM8739.pdf


