Does a fine line exist between regional and metastatic pelvic lymph nodes in rectal cancer—striking discordance between national guidelines and treatment recommendations by US radiation oncologists
Introduction
Approximately 40,000 patients are diagnosed with rectal cancer in the United States annually (1). The standard of care for locally advanced Stage II (T3–T4, N0, M0) and Stage III (Tany, N+, M0) rectal cancer, based on the results of a randomized German rectal trial (2), is preoperative chemoradiation therapy, followed by total mesorectal excision (TME) and additional adjuvant chemotherapy—with the goal of achieving greater than 60% of 5-year disease-free survival. In contrast, patients with metastatic disease are treated less aggressively, with the goal of prolonging survival and decreasing disease-related symptoms, but rarely with a curative intent.
Pelvic lymph nodes (LNs) outside of the mesorectum—internal iliac, external iliac, obturator, and common iliac (3)—are termed lateral pelvic lymph nodes (LPLNs). Rectal cancer with involved LPLNs is managed differently in the US compared to several countries in Asia. In the US, the American Joint Committee on Cancer (AJCC) defines only the internal iliac LN as regional (4), whereas external and common iliac LNs are considered sites of metastatic disease (5). On the other hand, in Japan all LPLNs are considered regional and patients are treated with curative intent (6).
Based on the MERCURY trial (7), as many as 10% of patients diagnosed with non-metastatic rectal cancer are found to have suspicious pelvic lymph nodes on diagnostic pelvic MRI. Current National Comprehensive Cancer Network (NCCN) guidelines recommend the inclusion of the primary tumor with pre-sacral and internal iliac LNs in the radiation treatment fields, whereas management of external and common iliac LNs is not specifically discussed, leading clinicians to assume that no chemoradiation therapy should be used in the management of rectal cancer patients with clinical involvement of these non-regional LNs (8). At the same time, radiation oncologists (ROs) routinely treat external and common iliac LNs in patients with other various pelvic malignancies. Therefore, we hypothesized that despite the current AJCC staging and NCCN guidelines, some ROs in the United States may approach patients with involvement of any LPLNs—both regional and non-regional—with curative intent. Institutionally, we coined the term “Stage 3.5” for rectal cancer patients with involved LPLNs—to highlight the uncertainty surrounding proper management of these patients.
Methods
Survey instrument development and data collection
We designed an online survey using REDCap software licensed by the Oregon Clinical and Translational Research Institute (OCTRI). The study was approved by the Oregon Health & Science University Institutional Review Board. The survey consisted of 14 questions pertaining to respondents’ demographics and use of imaging modalities. The online survey was sent anonymously by the REDCap data collecting software to 6,949 potential participants. Email invitations were sent in batches on November 16th and 17th of 2016 and a single reminder email was sent on November 30th, 2016.
Statistical analysis
Respondent characteristics (years in practice, practice setting, region of practice, number of rectal patients treated per year, and preferred utilization of imaging modalities) were tested for associations with respondents’ self-assessed approach to Stage 3.5 rectal cancer using Chi-squared or Fisher’s Exact test, as indicated. A P of less than 0.05 was defined as statistically significant. Staging 75% or more of rectal cancer patients with a given imaging modality was defined as high utilization. R [version 3.3.3 (2017-03-06)] was used for all data analysis.
Results
Respondent characteristics
Of the 6,949 email addresses, many belonged to the same physicians, who were registered in our database with both personal and institutional email accounts, making the determination of the response rate highly inaccurate. We received 337 failed/undelivered automatic responses, seven non-applicable/ineligible responses and 220 completed responses. The characteristics of these 220 individuals are summarized in Table 1. Sixty percent of respondents have practiced over 10 years since completion of residency training, 61% work in private practice, and 55% treat 10 or fewer patients with rectal cancer per year.

Full table
Recommendations regarding biopsy of pelvic LNs
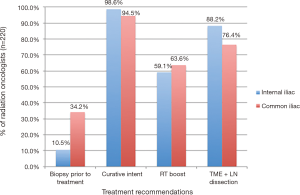
Among respondents, 10.5% recommend biopsy of the clinically involved internal iliac LN and 34.2% for the common iliac LN (Figure 1). A practice with a higher volume of rectal cancer patients, defined as seeing more than ten patients per year, was associated with a lower likelihood of internal LN biopsy recommendation (P=0.019) and a trend of lower likelihood of external LN biopsy recommendation (P=0.054). High MRI utilizers were also less likely to recommend the biopsy of the internal LN (P=0.010), but not of the common LN (P=0.365), as seen in Table 2.
Full table
Curative intent for rectal cancer patients with involved LPLNs need treatment intensification
There are 98.6% and 94.5% of respondents who approach rectal cancer patients with involved internal and common iliac LNs with curative intent, respectively, as shown in Figure 1. Among these respondents, 51.2% and 47.8% recommend combination of RT boost and pelvic lymph node dissection for involved internal iliac and common iliac LNs, respectively.
Only 3.7% and 5.3% of those respondents who approach patients with involved internal and common iliac LNs with curative intent, respectively, do not recommend any treatment intensification beyond the standard of care for locally advanced rectal cancer.
High PET/CT utilizers were more likely to recommend RT boost to involved common iliac LNs (70.9% vs. 57.3%, P=0.036), as shown in Table 3.
Full table
Among our respondents, 88.2% recommend surgical lymph node dissection of internal iliac LNs and 59.1% recommend RT boost, as shown in Figure 1. For common iliac LNs these percentages are 76.4% and 63.6% for surgical vs. RT boost treatment intensification, respectively. Respondents who practice in private clinics are more likely to recommend surgical dissection of involved common iliac LNs at the time of TME than academic physicians (81.5% vs. 62.8%, P=0.024), with a trend for a similar association for surgical management of internal iliac LN (P=0.09) as shown in Table 4.
Full table
Discussion
Biopsy confirmation of clinically involved LPLNs in patients with rectal cancer
Previous studies have discouraged LN biopsy in rectal cancer patients given the high rate of false negatives (3–12%) in the setting of T3/T4 primary rectal tumor (9). However, there is a significant lack of clinical experience and guidance regarding biopsy of clinically suspicious lateral pelvic lymph nodes in these patients. This is becoming a common clinical scenario with increasing pelvic MRI utilization; among patients diagnosed with rectal cancer who have no evidence of distant metastases, as many as 10% are found to have clinically suspicious LPLNs (7). Among our respondents, those who frequently evaluate rectal cancer patients, defined as practice with more than ten rectal cancer patients per year, felt more comfortable treating patients without biopsy of suspicious internal LNs. The same association was noted for respondents who frequently utilize pelvic MRI for staging of rectal cancer. Further clinical studies are critical to determine if pathological confirmation of suspicious LPLNs would allow clinicians to personalize treatment for patients, without excess toxicity. Novel imaging modalities, such as USPIO-MRI, are currently being evaluated as non-invasive modalities with higher sensitivity and specificity over routine pelvic MRI (Clinicaltrial.gov identifier for MRI-USPIO rectal cancer protocol at OHSU Knight Cancer Institute: NCT03280277).
Dramatic discordance between NCCN guidelines and current practice patterns of US ROs
Our survey results show that practicing ROs in the United States almost uniformly approach rectal cancer patients with both internal (Stage III) and common (Stage IV) iliac LNs with curative intent, despite the current national guidelines recommendations based on AJCC staging criteria. Clinical evidence from Asian countries that embrace curative management of rectal cancer patients with LPLNs supports surgical dissection of involved pelvic lymph nodes. High incidence of LPLN metastases was reported after preoperative chemoradiation therapy—which primarily targets mesorectum and internal pelvic lymph nodes. Addition of LPLN dissection to TME was shown to decrease 3-year local recurrence rate from 7.1% down to 2.7% in one study (6) and even revealed a statistical improvement in 5-year local-recurrence-free survival in another study (10).
Our survey shows that the great majority of US ROs recommend surgical dissection of involved LPLNs, both regional (internal LN) and non-regional (common LN). Unfortunately, we did not query our respondents whether they were aware of the Asian surgical literature to determine whether their recommendations were based on this clinical evidence or based on extrapolation from oncological management of other pelvic malignancies. Over half of respondents also recommend, and likely use in their practice, dose-intensification to the involved LPLNs by using RT boost. It is imperative to determine the appropriate management of these patients through prospective clinical trials. Until then, the national leaders in the field of rectal cancer need to draft a consensus statement regarding the appropriate management, to help practicing physicians evaluate and manage these patients safely and effectively.
Limitations
The greatest limitation of our study is a low response rate. It is likely that response bias could have introduced a misrepresentation into actual current patterns of care in the United States. Unfortunately, all claim-based retrospective studies would not be able to capture the most up-to-date snapshot of real-time practice patterns and would require several years of data capture and analysis to shed light on this important question.
Conclusions
Our survey-based analysis of current practice patterns among US ROs reveals a dramatic discordance between the national guidelines and treatment recommendations for Stage 3.5 rectal cancer patients—those with involved LPLNs, but in the absence of distant metastases. The overwhelming majority of practicing ROs approach these patients with curative intent. Moreover, against the national guidelines that recommend treatment de-intensification in the non-curative setting, most practicing ROs in the US recommend treatment intensification, in the form of involved pelvic LN dissection, RT boost or both. The management of Stage 3.5 rectal cancer patients with involved LPLNs is currently not based on robust clinical evidence, and prospective clinical studies are greatly needed to establish the most appropriate management of these patients. Until this data is known, consensus guidelines must be issued by clinician-leaders in the field of rectal cancer to guide practicing oncologists to deliver safe and effective treatment to patients with Stage 3.5 rectal cancer.
Acknowledgements
We thank the respondents who have taken the time to participate and complete our survey.
Funding: This work was supported by OHSU REDCap [1 UL1 RR024140 01].
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board of Oregon Health and Science University (IRB protocol 11149), and the requirement to obtain informed consent from survey respondents was waived.
References
- Siegel RL, Miller K, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiation therapy in rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Yagi R, Shimada Y, Kameyama H, et al. Clinical significance of extramural tumor deposits in the lateral pelvic lymph node area in low rectal cancer: A retrospective study at two institutions. Ann Surg Oncol 2016;23:552-8. [Crossref] [PubMed]
- Edge S, Byrd DR, Compton CC, et al. AJCC cancer staging manual, 7th ed. NY: Springer-Verlag, 2010.
- American Joint Committee on Cancer. Colon and Rectum Cancer Staging 7th ed. Available online: https://cancerstaging.org/references-tools/quickreferences/Documents/ColonMedium.pdf
- Akiyoshi T, Watanabe T, Miyata S, et al. Results of a Japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer: Is it regional or distant disease? Ann Surg 2012;255:1129-34. [Crossref] [PubMed]
- Taylor FG, Quirke P, Heald RJ, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol 2014;32:34-43. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Rectal Cancer Version 3.2017 reference information. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
- Cahill RA. What's wrong with sentinel node mapping in colon cancer? World J Gastroenterol 2007;13:6291-4. [Crossref] [PubMed]
- Fujita S, Mizusawa J, Kanemitsu Y, et al. Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial. Ann Surg 2017;266:201-7. [Crossref]


