|
Original Article
Association of Technetium99m MAG-3 Renal Scintigraphy with Change in Creatinine Clearance Following Chemoradiation to the Abdomen in Patients with Gastrointestinal Malignancies
Kilian Salerno May1, Gary Y Yang2, Nikhil I Khushalani3, Rameela Chandrasekhar4, Gregory E Wilding4, Leayn Flaherty1, Harish K Malhotra1, Richard C Russo1, John C Warner5, Johnny C Yap1, Renuka V Iyer3, Chukwumere E Nwogu6, Saikrishna S Yendamuri6, John F Gibbs6, Hector R Nava6, Dominick Lamonica5, Charles R Thomas Jr7
1Department of Radiation Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA; 2Department of Radiation Medicine, Loma Linda University Medical Center, Loma Linda, California, USA; 3Department of Medical Oncology, Roswell Park Cancer Institute, Buffalo, New York, USA; 4Department of Biostatistics, Roswell Park Cancer Institute, Buffalo, New York, USA; 5Department of Nuclear Medicine, Roswell Park Cancer Institute, Buffalo, New York, USA; 6Department of Surgical Oncology, Roswell Park Cancer Institute, Buffalo, New York, USA; 7Department of Radiation Medicine, Oregon Health & Science University, Portland, Oregon, USA
Correspondence to: Gary Yang MD, Department of Radiation Medicine, Loma Linda University Medical Center. 11234 Anderson Street, B121, Loma Linda, CA 92354. Tel: 909-508-4280; Fax: 909-558-4083. Email: gyang@llurm.org
|
|
Abstract
Background: Information on differential renal function following abdominal chemoradiation is limited. This study evaluated the association between renal function as measured by biochemical endpoints and scintigraphy and dose volume parameters in patients with gastrointestinal malignancies.
Materials and methods: Patients who received abdominal chemoradiation between 2002 and 2009 were identified for this study. Technetium99m MAG-3 scintigraphy and laboratory data were obtained prior to and after chemoradiation in 6 month intervals. Factors assessed included age, gender, hypertension, diabetes, and dose volume parameters. Renal function was assessed by biochemical endpoints and renal scintigraphy.
Results: Significant reductions in relative renal function of the primarily irradiated kidney and creatinine clearance were seen. Split renal function decreased from 49.75% pre-radiation to 47.74% and 41.28% at 6-12 months and >12 months postradiation (p=0.0184). Creatinine clearance declined from 90.67ml/min pre-radiation to 82.23ml/min and 74.54ml/min at 6-12 months and >12 months post-radiation (p<0.0001). Univariate analysis of patients who had at least one post-radiation renogram showed the percent volumes of the primarily irradiated kidney receiving ≥ 25 Gy (V25) and 40 Gy (V40) were significantly associated with ≥5% decrease in relative renal function (p=0.0387 and p=0.0438 respectively).
Conclusion: Decline in split renal function using Technetium99m MAG-3 scintigraphy correlates with decrease in creatinine clearance and radiation dose-volume parameters following abdominal chemoradiation. Change in split perfusion can be detected as early as 6 months post-radiation. Scintigraphy may provide early determination and quantification of subclinical renal injury prior to clinical evidence of nephropathy.
Key words Renal Function, Renal Scintigraphy, Creatinine Clearance, Chemoradiation, Gastrointestinal Malignancies
J Gastrointest Oncol 2010; 1: 7-15. DOI: 10.3978/j.issn.2078-6891.2010.001
|
|
Introduction
Gastrointestinal malignancies are frequently treated with combined modality therapy using chemoradiation. Current abdominal radiation uses volumetric data from CT based planning to better define targets and organs at risk. One or both of the kidneys often lie in close proximity to target structures. As the kidneys are inherently radiosensitive and renal tolerance limits are often less than prescribed therapeutic doses, the kidneys are major dose limiting structures in abdominal radiation treatment fields. Progressive renal dysfunction following abdominal radiation has been reported (1-13). Emami et al described the probability of developing normal tissue complications and suggested organ tolerance limits based on volume of organ irradiated to various doses (14). For kidney, the tolerance limits for 5% probability of complications at 5 years (TD 5/5) are 23 Gy for whole organ, 30 Gy for 2/3 volume, and 50 Gy to 1/3 volume. The Emami tolerances do not specifically address the relative contribution of each kidney to overall renal function. Split renal function is commonly assessed prior to abdominal radiation. Split renal function can be measured using renal scintigraphy with each kidney’s relative function expressed as a percentage of total function. Assessment of the relative contribution of each kidney to overall renal function by renogram may guide radiation treatment planning and design of shielding for renal sparing.
This study evaluated renal function prior to and following abdominal radiation with concurrent chemotherapy in the treatment of gastrointestinal malignancies. The association between split function on Technetium99m MAG-3 renal scintigraphy, change in creatinine clearance, and radiation dose volume parameters was analyzed.
|
|
Methods and materials
Patient Selection
Patients with gastrointestinal malignancies treated with abdominal chemoradiation between 2002 and 2009 were identified. Patients were included in this analysis if they received concurrent chemotherapy and three-dimensional conformal abdominal radiation, had at least one kidney included in the radiation treatment fields, had pre-radiation renal scintigraphy performed, received at least 20 Gy, and had laboratory data and dosimetric parameters available for review.
Chemoradiation
All patients underwent CT simulation. Three-dimensional conformal radiation treatment planning was performed using Theraplan Plus treatment planning system (MDS Nordion, Ottawa, Ontario, Canada) and Eclipse Treatment Planning System (Varian Medical Services, Palo Alto, CA, USA). Abdominal radiation was delivered on linear accelerators using 6-23 MV photons. Dose and field arrangements varied by primary site. Targets and organs at risk were contoured. Treatment plans were designed to encompass the primary target and areas at risk with margin. Planning dose constraints for organs at risk were: heart 1/3 volume not to exceed 60 Gy and 2/3 volume not to exceed 45 Gy; liver 1/3 volume not to exceed 50 Gy and 2/3 volume not to exceed 35 Gy; and kidneys(combined) 1/3 volume not to exceed 50 Gy and 2/3 volume not to exceed 30 Gy.
The kidney that received the greater mean kidney dose was defined as the primarily irradiated kidney. The kidney that received the lesser mean kidney dose was defined as the nonprimarily irradiated kidney.
All patients received concurrent chemotherapy. Few patients received chemotherapy prior to radiation and most patients received further chemotherapy following radiation.
Renal Scintigraphy
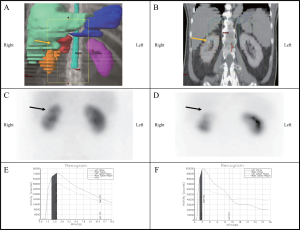
All patients received intravenous hydration prior to intravenous injection of 6 mCi of Technetium99m MAG-3. Renal scintigraphy was performed with the patient in the supine position and images were obtained in the posterior projection. Sequential flow images were obtained for quantitative analysis of the renogram, initially taken as 1 second per frame for the first minute and then as 30 seconds per frame for the next 30 minutes. The posterior images were obtained using a 64 x 64 matrix on a large field of view gamma camera with low energy collimators. Split uptake of left to right relative function was measured over the initial 2-3 minute interval post injection and was determined using the time–activity curve generated after the acquisition was completed.
Endpoints
Endpoints analyzed included relative renal function on renal scintigraphy, biochemical endpoints, and dose volume parameters. Change in split renal function was evaluated by comparison of the relative contribution of each kidney on renogram. Biochemical endpoints used to assess change in renal function included serum creatinine and creatinine clearance. Creatinine clearance was calculated using the Cockcroft-Gault formula: {{(140-age) x (weight in kilograms)} / (72 x serum creatinine)} (15). This value was adjusted for female gender by multiplying the creatinine clearance x 0.85. Renal scintigraphy, laboratory data, and biochemical endpoints were determined prior to and after radiation in 6 month intervals.
Statistical Analysis
Statistical analyses for categorical variables were performed using Fisher’s exact test while continuous variables were analyzed using the Wilcoxon non-parametric test with exact p-values obtained using Monte-Carlo estimates. Change in outcome variables over time were assessed using a repeated measures model. To account for missing data, a pattern mixture model was used. Values for continuous variables are given as mean (standard deviation) while values for categorical data are specified as number (percentage). Statistical analysis was performed using SAS Statistical analysis software version 9.1.3 (SAS Institute Inc, Cary, NC, USA). A nominal significance level of 0.05 was used.
|
|
Results
Patient and Treatment Characteristics
One hundred thirty six patients were identified who received abdominal radiation with concurrent chemotherapy, had renal scintigraphy performed prior to radiation, received at least 20 Gy, and had dose volume parameters and mean kidney doses available for analysis. Median patient age was 64 years. The majority of primary disease sites were pancreas and periampullary (75.7%). Median follow up was 9.6 months. Patient characteristics are provided in Table 1.
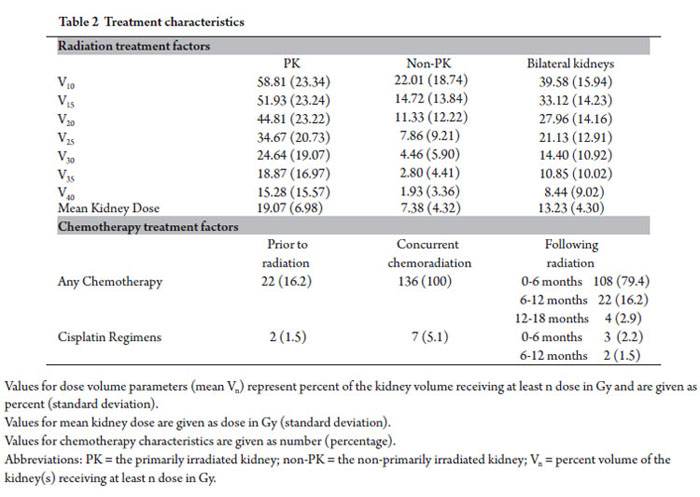
Median radiation dose delivered to target was 50.4 Gy (range 21.6-55.8 Gy). Mean kidney dose of the primarily irradiated kidney was 19.07 Gy. Additional radiation dose volume parameters are presented in Table 2.
All 136 patients included in this study received concurrent chemotherapy. Twenty two patients (16.2%) received chemotherapy prior to radiation; 108 patients (79.4%) received additional chemotherapy 0-6 months following RT; 22 patients (16.2%) received chemotherapy 6-12 months following radiation; and 4 (2.9%) patients received further chemotherapy 12-18 months following radiation. Fourteen patients (10.3%) received cisplatin containing regimens at any time point evaluated in this study. One hundred and twenty two patients (89.7%) did not receive any cisplatin as a part of their chemotherapeutic regimens. Specific chemotherapy regimens used pre-radiation, concurrently, and post-radiation varied given the different primary sites included in this analysis. The vast majority of patients received 5-fluorouracil, capecitabine, and/or gemcitabine based regimens. Chemotherapy characteristics are summarized in Table 2.

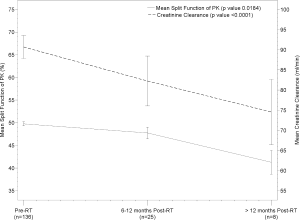
Change in Renal Scintigraphy and Renal Function over Time
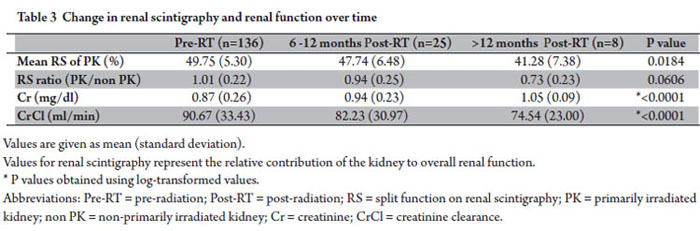
Progressive change in renal function was assessed by split renal function on scintigraphy, creatinine, and creatinine clearance from baseline prior to radiation and then in 6 month intervals following radiation. Patients with mean kidney doses, laboratory data, and split renal function data per scintigraphy at each time point were included in this analysis. Results are presented in Table 3.
Split renal function of the primarily irradiated kidney decreased from 49.75% pre-radiation, to 47.74% and 41.28% at 6-12 months and >12 months following radiation (p=0.0184). The ratio of split renal function of the primarily irradiated kidney to the non-primarily irradiated kidney declined over time from baseline equal split function ratio of 1.01 prior to radiation. Creatinine increased from a mean value of 0.87 mg/dL pre-radiation, to 0.94 mg/dL and 1.05 mg/dL at 6-12 months and >12 months following radiation (p<0.0001). Creatinine clearance decreased from 90.67ml/min pre-radiation, to 82.23 ml/min and 74.54 ml/min at 6-12 months and >12 months following radiation (p<0.0001). No patients developed malignant hypertension or other signs of clinical nephropathy. Change in split renal function of the primarily irradiated kidney and creatinine clearance over time is shown in Figure 1.
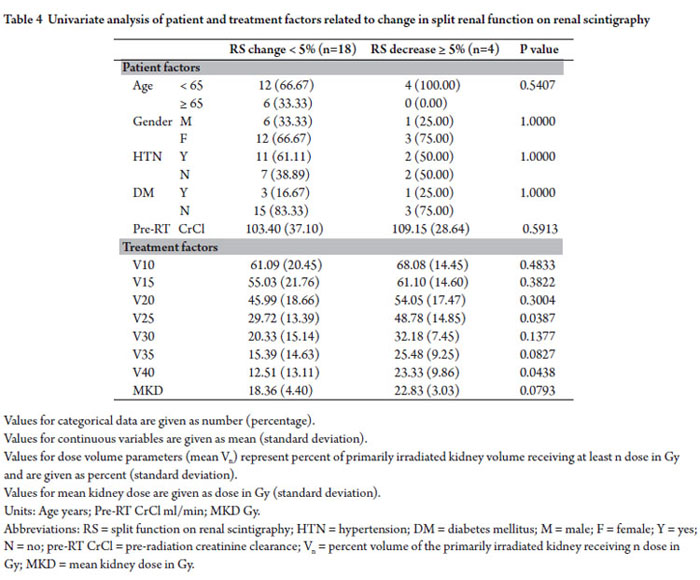
Univariate Analysis of Patient and Treatment Factors Related to Change in Split Renal Function on Renal Scintigraphy
Twenty two patients were identified who had <5% increase, no change, or decrease split function on renogram obtained 6-12 months following radiation and had complete dose volume parameters available for review. Of these, 18 of the patients (82%) had change in the relative renal function of the primarily irradiated kidney of <5% and 4 patients (18%) had decreases of ≥5%. No patient related factors were found to be associated with decrease in split renal function of the primarily irradiated kidney. Percent volumes of the primarily irradiated kidney receiving ≥25 Gy (V25) and 40 Gy (V40) were associated with decrease of ≥5% relative renal function (p=0.0387 and p=0.0438 respectively). Difference in mean kidney dose of the primarily irradiated kidney between patients with <5% change in split renal function and those with ≥5% decrease trended towards significance (p=0.0793)(Table 4).
|
|
Discussion
Split renal function of the primarily irradiated kidney and creatinine clearance were found to significantly decrease over time following abdominal chemoradiation. Progressive decline in relative renal function and biochemical endpoints were seen as early as 6 months following completion of radiation. V25 and V40 were shown to correlate with ≥5 % decrease in relative renal function of the primary irradiated kidney at 6-12 months post-radiation.
The literature available on progressive change in renal function following abdominal chemoradiotherapy in the treatment of gastrointestinal malignancies is limited. Renal effects of radiation are dose and volume dependent (4). Renal tolerances per Emami et al predict the likelihood of normal tissue complications based on volume of tissue irradiated to specific doses (14). Current dose tolerance limits do not specifically consider the relative function of each kidney and its contribution to global renal function (14,16). Pathophysiologic mechanisms for radiation induced renal injury include glomerular and tubular damage and disruption of renal microvasculature (4,7,10). Subclinical evidence of renal injury can be observed within 6-12 months following radiation (1-3,12,13). A longer latency period is needed for development of symptomatic nephropathy (2,4,7,10). Renal toxicity can be detected by functional and biochemical endpoints in advance of presentation of clinical symptoms (5,6,8,9,11-13). Biochemical endpoints such as serum creatinine and creatinine clearance are reflective of global renal function. Serum creatinine is the most commonly used biochemical measure of renal function in clinical practice; however significant decline in glomerular filtration rate can occur prior to rise in creatinine (17,18). Creatinine clearance is an estimate of glomerular filtration rate and a more sensitive measurement of renal function than creatinine (19). Creatinine clearance is measured by 24 hour urine collection. An estimated creatinine clearance can be calculated by various formulas including the Cockcroft-Gault equation (15). While estimated creatinine clearance is not fully concordant with measured creatinine clearance, such formulas are frequently used in clinical practice as the clinical and laboratory data points needed for calculation are readily available on most patients (20,21). Nuclear renography can provide additional clinically relevant information on renal function and can be used to determine glomerular filtration rate and differential renal function (18,22,23). Renal clearance by scintigraphy is an accurate estimate of glomerular filtration rate and correlates well with creatinine clearance measured by 24 hour urine collection (24,25). Assessment of each kidney’s relative contribution to global renal function can be done by both scintigraphy and biochemical endpoints. Techniques for measurement of unilateral creatinine clearance are available but are invasive and not practical for routine use. Correlation between split renal function as determined by renogram and lateralized creatinine clearance has been shown (26). Renal scintigraphy can provide accurate quantitative determination of relative renal function less invasively (22,23,27). The normal range for symmetric split function of each kidney is between 45-55% and changes of ≥5% are considered significant (28-30). Renal scintigraphy can detect post radiotherapy renal damage prior to creatinine elevation (17,31-33). LeBourgeois and colleagues reported their findings on the renal effects of splenic irradiation in lymphoma patients using 197Hg neohydrin scintigrams (33). Reduced uptake of radioisotope in the irradiated kidney was detected by the eighth month following radiation, with stabilization after the twentieth month, but not recovery. Radiation induced injury to the kidney was prospectively studied by Dewit et al using scintigraphic and biochemical endpoints (6). Significant progressive renal toxicity was seen following abdominal radiation. In patients who received the highest doses to the entire left kidney, renal function assessed by scintigraphy decreased by 60-70% after 3-5 years whereas creatinine clearance decreased by 20%. In patients in who part of the kidney was shielded, relative renal function decreased by 20-25% at 5 years. In a prospective analysis of relative renal function and late toxicity in patients with gastric cancer who received postoperative chemoradiation, dose volume parameters for the left kidney were predictive of worse subsequent decline in split renal function (11). Jansen et al observed a progressive decline in left renal function with 11% decrease at 6 months and 52% decline at 18 months post-radiation. In patients with non primary gastrointestinal malignancies treated with chemoradiation, Kost and colleagues prospectively investigated the dose effect on renal damage using functional and biochemical endpoints (9). Decline in renal function on static scintigraphy was seen in 23% of patients. The extent of scintigraphic change was related to dose and volume irradiated and changes were observed even at relatively low doses (<10Gy). Decline of the relative contribution of the irradiated kidney to overall renal function was detected at one year post-radiation and progressively decreased to 35-40% at 3 years. The influence of chemotherapy on renal toxicity was not specifically analyzed given small patient numbers and the variety of chemotherapeutic regimens included. Use of nephrotoxic chemotherapeutic agents such as cisplatin with abdominal radiation has been shown to reduce renal tolerance and potentiate renal toxicity (34-37). All patients in our study received concurrent chemotherapy and most received additional systemic therapy following radiation. As only 10% patients received cisplatin containing regimens, we did not analyze the use of cisplatin as a predictor for subsequent renal dysfunction. Nor did we analyze the influence of other chemotherapeutic agents specifically given the variability of regimens used. We further evaluated patients who had at least one renogram obtained 6-12 months post-radiation, biochemical data, and dose volume parameters available for factors associated with subsequent decline in split renal function of the primarily irradiated kidney. Of patients identified, three patients demonstrated increases greater than 5% in relative renal function of the primarily irradiated kidney from baseline renogram. This observation is unexpected following radiation and the explanation for increase in split renal function is likely multifactorial. These patients were not included in the subsequent analysis.
In patients with < 5% increase, stable, or decreased split renal function following radiation, no patient related factors were found to be associated with decrease in relative renal function of the primarily irradiated kidney. Treatment related factors significantly associated with decrease of ≥5% relative renal function on univariate analysis included V25 and V40. The mean kidney dose of the primarily irradiated kidney trended toward significance. Although our study was not able to identify other factors associated with decrease in relative renal function following abdominal radiation, it is likely that additional dose volume parameters are involved as they are interrelated. The limited sample size of patients with postradiation renograms available for analysis likely precluded our ability to detect these differences.
Our study is consistent with others in showing progressive decline in renal function as measured by functional scintigraphic imaging and biochemical endpoints (6,9,11). In contrast to Kost et al who noted decline after one year, we observed decreases in relative renal function and biochemical endpoints as early as 6 months post-radiation. We observed significant decline in both relative renal function of the primarily irradiated kidney detected on scintigraphy and global renal function as measured by creatinine clearance following abdominal chemoradiation. Post- radiation renography in combination with biochemical measures may allow for early identification and assessment of patients at greater risk for developing clinical manifestations of radiation nephropathy. As radiation induced renal injury is progressive, it is likely that the functional impairments observed in this study will increase over time. With longer follow up, further correlation between changes detected on renal scintigraphy, biochemical endpoints, and radiation dose volume parameters may be observed.
|
|
Conclusions
Decline in split renal function using Technetium99m MAG-3 scintigraphy correlates with decrease in creatinine clearance and radiation dose-volume parameters following abdominal chemoradiation. Change in split perfusion can be detected as early as 6 months post-radiation. This observation suggests post-radiation scintigraphy may allow for early determination and quantification of subclinical renal injury prior to development of clinical nephropathy.
|
|
Refrences
- Kunkler PB, Farr R, and RW Luxton. The limit of renal tolerance to xrays. Br J Radiol 1952; 25:190-201.[LinkOut]
- Luxton RW. Radiation nephritis. Q J Med 1953; 22: 215-242.[LinkOut]
- Thompson, PL, Mackay IR, Robson GSM, and AJ Wall. Late radiation nephritis after gastric x-irradiation for peptic ulcer. Q J Med 1971; 40:145-157.[LinkOut]
- Krochak RJ and DG Baker. Radiation nephritis: clinical manifestations and pathophysiologic mechanisms. Urology 1986; 27: 389-393.[LinkOut]
- Willett CG, Tepper JE, Orlow EL, and WU Shipley. Renal complications secondary to radiation treatment of upper abdominal malignancies. Int J Radiat Oncol Biol Phys 1986; 12: 1601-1604.[LinkOut]
- Dewit L, Anninga JK, Hoefnagel CA, and WJ Nooijen. Radiation injury in the human kidney: a prospective analysis using specific scintigraphic and biochemical endpoints. Int J Radiat Oncol Biol Phys 1990; 19: 977-983.[LinkOut]
- Cassady JR. Clinical Radiation Nephropathy. Int J Radiat Oncol Biol Phys 1995; 31: 1249-1256.[LinkOut]
- Maor, MH, North LB, Cabanillas FF, Ames, AL, Hess, MA, and JD Cox. Outcomes of high-dose unilateral kidney irradiation in patients with gastric lymphoma. Int J Radiat Oncol Biol Phys 1998; 41: 647-650.[LinkOut]
- Kost S, Dorr W, Keinert K, Glaser FH, Endert G, and T Herrmann. Effect of dose and dose-distribution in damage to the kidney following abdominal radiotherapy. . Int J Radiat Oncol Biol Phys 2002; 78: 695-702.[LinkOut]
- Cohen EP and MEC Robbins. Radiation nephropathy. Semin Nephrol 2003; 23: 486-499.[LinkOut]
- Jansen EPM, Saunders MP, Boot H, et al. Prospective study on late renal toxicity following postoperative chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys 2007; 67: 781-785.[LinkOut]
- May KS, Khushalani NI, Chandrasekhar R, et al. Analysis of clinical and dosimetric factors associated with change in renal function in patients with gastrointestinal malignancies after chemoradiation to the abdomen. Int J Radiat Oncol Biol Phys 2010; 76: 1193-1198.[LinkOut]
- Yang GY, May KS, Iyer RV, et al. Renal atrophy secondary to chemoradiotherapy of abdominal malignancies. Int J Radiat Oncol Biol Phys. In press. doi:10.1016/j.ijrobp.2009.07.1744.[LinkOut]
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991; 21: 109-122.[LinkOut]
- Cockcroft DW and MH Gault. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16: 31-41.[LinkOut]
- Milano MT, Constine LS, and P Okunieff. Normal tissue tolerance dose metrics for radiation therapy of major organs. Semin Radiat Oncol 2007; 17: 131-140.[LinkOut]
- Brochner-Mortensen J. Current status on assessment and measurement of glomerular filtration rate. Clin Physiol 1985; 5: 1-17.[LinkOut]
- Haufe SE, Riedmuller K, and U Haberkorn. Nuclear medicine procedures for the diagnosis of acute and chronic renal failure. Nephron Clin Pract 2006; 103: c77-84.[LinkOut]
- Leoncini G, Viazzi, Parodi D, et al. Creatinine clearance and signs of endorgan damage in primary hypertension. J Hum Hypertens 2004; 18: 511-516.[LinkOut]
- Raj GV, Iasonos A, Herr H, and SM Donat. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 2006; 24: 3095-3100.[LinkOut]
- Hurria A, Hurria A, Brogan K, et al. Effect of creatinine clearance on patterns of toxicity in older patients receiving adjuvant chemotherapy for breast cancer. Drugs Aging 2005; 22: 785-791.[LinkOut]
- Taylor Jr A and JV Nally. Clinical applications of renal scintigraphy. AJR 1995; 164: 31-41.[LinkOut]
- Taylor A. Radionuclide renography: a personal approach. Semin Nuc Med 1999; 24: 102-127.[LinkOut]
- Petersen LJ, Petersen JR, Talleruphuus U, et al. Glomerular filtration rate estimated from the uptake phase of 99mTc-DTPA renography in chronic renal failure. Nephrol Dial Transplant 1999; 14: 1673-1678.[LinkOut]
- Esteves FP, Halkar RK, Issa MM, Grant S, and A Taylor. Comparison of camera-based 99mTc-MAG3 and 24-hour creatinine clearances for evaluation of kidney function. AJR 2006; 187: W316-W319.[LinkOut]
- Mindrup SR, Cooper CS, Hodroff MA, and CE Hawtrey. Comparison of relative renal function by renal scintigraphy and lateralized creatinine clearance in children with bilateral vesicoureteral reflux. Urology 2003; 61: 816-819.[LinkOut]
- Prigent A, Cosgriff P, Gates GF, et al. Consensus report on quality control of quantitative measurements of renal function obtained from the renogram: international consensus committee from the scientific committee of radionuclides in nephrolourology. Semin Nuc Med 1999; 24: 146-159.[LinkOut]
- Piepsz A, Coralinha P, Gordon I, et al. Guidelines for 99mTc-DMSA scintigraphy in children. Eur J Nucl Med 2001; 28: BP37-BP41.[LinkOut]
- Piepsz A, and HR Ham. Pediatric applications of renal nuclear medicine. Semin Nuc Med 2006; 36: 16-35.[LinkOut]
- Piepsz A, Ismaili K, Hall M, Collier F, Tondeur M, and H Ham. How to interpret a deterioration of split function? Eur Urol 2005; 47: 686-690.[LinkOut]
- Quinn JL, Meschan I, Blake DD, and RL Witcovski. The usefulness of the radioisotopic renogram in radiation therapy. Radiology 1962; 78: 266-268.[LinkOut]
- Davey PW, Hamilton JD, and RW Luxton. The limit of renal tolerance to Xrays: an investigation into renal damage occurring following the treatment of tumors of the testis by abdominal baths. Br J Radiol 1952; 25: 190-201.[LinkOut]
- LeBourgeois JP, Meignan M, Parmentier C, and M Tubiana. Renal consequences of irradiation of the spleen in lymphoma patients. Br J Radiol 1979; 52: 56-60.[LinkOut]
- Tarbell NJ, Guinan EC, Niemeyer C, et al. Late onset of renal dysfunction in survivors of bone marrow transplantation. Int J Radiat Oncol Biol Phys 1988; 15: 99-104.[LinkOut]
- Stewart FA, Oussoren Y, and H Bartelink. The influence of cisplatin on the response of mouse kidneys to multifraction irradiation. Radiother Oncol 1989; 15: 93-102.[LinkOut]
- Moulder JE and BL Fish. Effect of sequencing on combined toxicity of renal irradiation and cisplatin. NCI Monogr 1988; 6: 35-39.[LinkOut]
- Welz S, Hehr T, Kollmannsberger C, et al. Renal toxicity of adjuvant chemoradiotherapy with cisplatin in gastric cancer. Int J Radiat Oncol Biol Phys 2007; 69: 1429-1435.[LinkOut]
Cite this article as:
May K, Yang G, Khushalani N, Chandrasekhar R, Wilding G, Flaherty L, Malhotra H, Russo R, Warner J, Yap J, Iyer R, Nwogu C, Yendamuri S, Gibbs J, Nava H, Lamonica D, Thomas C Jr. Association of Technetium99m MAG-3 Renal Scintigraphy with Change in Creatinine Clearance Following Chemoradiation to the Abdomen in Patients with Gastrointestinal Malignancies. J Gastrointest Oncol. 2010;1(1):7-15. DOI:10.3978/j.issn.2078-6891.2010.001
|