Refining the management of resectable esophagogastric cancer: FLOT4, CRITICS, OE05, MAGIC-B and the promise of molecular classification
Introduction
Although no longer the most common cancer worldwide gastric cancer (GC) remains 1 of 8 major cancers that together account for more than 60% of total cancer cases and deaths worldwide (1). In 2012 nearly 1 million new cases of GC were diagnosed globally with an estimated 723,100 deaths, reflective of the high mortality rate associated with this disease. Nearly half of GC patients present with locoregional disease where surgical resection is potentially curative (2). However, the majority of GC patients relapse following resection and the 5-year survival rate across all stages remains poor at 25–30% (2,3). For stage IA GCs [American Joint Committee on Cancer (AJCC) 7th Edition] that are specifically confined to the mucosa (clinical T1a), well-differentiated, ≤2 cm, and non-ulcerated, most major guidelines recommend endoscopic resection as a treatment option in experienced centers (3-5). For stage IB–IIIC GC, gastrectomy is highly recommended though the timing, extent of surgery, particularly degree of accompanying nodal dissection has been the subject of controversy (3-5). Overall, combined modality therapies remain a global standard for ≥ stage II GC.
Although curative in a small subset of patients, surgery alone is associated with high recurrence rates, presumably owing to unrecognized micrometastatic disease driving metastatic recurrence. Neoadjuvant, perioperative, and adjuvant approaches explored over the last two decades have resulted in modest outcome improvements (2,3,6). However, there remains no universal standard of care for resectable GC (2,6). Geographic variations in surgical approach, access to tertiary centers, referral patterns, and phase III data representative of a given region have confounded global standardization. Observations from prior phase III datasets in resectable GC spawned a recent series of modern phase III trials, namely FLOT4, MAGIC-B, OE05, and CRITICS. Herein we review the key findings from these modern trials in the context of current practice. We highlight implications of molecular GC classification from collaborative efforts including The Cancer Genome Atlas (TCGA) and ACRG, and how incorporation may optimize patient selection, incorporate immunotherapy, and guide future studies to improve patient outcomes in locoregional GC.
Adjuvant chemoradiation (CRT)
The seminal Southwestern Oncology Group/Intergroup (SWOG/INT) 0116 was the first U.S. randomized-controlled, phase III clinical trial to demonstrate a survival benefit from adjuvant therapy in resected esophagogastric adenocarcinomas (7). Patients (n=603) with radically resected stage IB through IVM0 adenocarcinoma of the stomach or gastroesophageal junction (AJCC 3rd edition) were randomly assigned after surgery to either observation or adjuvant fluoropyrimidine-based CRT (7). RT was comprised of 45 Gy given as 1.8 Gy daily for 5 days per week for 5 weeks. The recommended surgical approach was a D2 lymph node dissection according to the Japanese Gastric Cancer Association guidelines entailing removal of all perigastric lymph nodes, celiac, splenic or splenic-hilar, hepatic artery, and cardial lymph nodes, with some exceptions based on the primary tumor location in the stomach (4). However, among the INT0116 subjects the majority (54%) underwent a D0 dissection (removal of less than the Japanese N1 nodal stations), followed by patients with D1 (36%) and D2 (10%) lymphadenectomies. At a median of 5 years of follow-up, the authors reported an overall survival (OS) of 36 months in CRT arm compared to 27 months in the surgery-only cohort (HR 1.35; 95% CI: 1.09–1.66; P=0.005) (Table 1). Longer term follow up has confirmed benefit from adjuvant chemoradiotherapy with improvements in OS (HR 1.32; 95% CI: 1.10–1.60; P=0.0046) and relapse-free survival (RFS) (HR 1.51; 95% CI: 1.25–1.83; P<0.001) (10). Notably, it is difficult to draw definitive conclusions from benefit of CRT in those receiving D2 lymphadenectomy given the small number (n=54) of patients included, and the concept of CRT “overcoming” suboptimal surgery is a frequent INT0116 criticism.

Full table
While the INT0116 established the dominant US approach to resected GC after publication in 2001, the differing Asian surgical patterns and INT0116 findings left important unanswered questions. The phase III ARTIST trial sought to clarify whether adjuvant CRT benefits GC patients after D2 lymph node dissection. This study randomly allocated patients with completely resected stage IB (with exception of pathologic T2aN0) through IV (M0) gastric adenocarcinoma (AJCC 6th edition staging) to receive capecitabine with cisplatin (XP) or XP followed by radiotherapy with concurrent capecitabine followed by further XP (XP/XRT/XP) (8) (Table 1). All patients were enrolled by participating centers in South Korea, China, and Taiwan. In the XP cohort, 172 of 228 patients (75.4%) successfully completed 6 cycles of adjuvant capecitabine 2,000 mg/m2 divided twice daily on days 1 to 14 and cisplatin 60 mg/m2 on day 1 (every 3 week cycles) while in the XP/XRT/XP cohort, 188 of 230 (81.7%) patients successfully completed 2 cycles of adjuvant capecitabine and cisplatin followed by 45 Gy and capecitabine 1,650 mg/m2 divided twice daily for 5 weeks followed by 2 more cycles of capecitabine and cisplatin. The authors reported no statistically significant difference in disease-free survival (DFS) with the addition of post-operative radiation therapy to XP [3-year DFS 78.2% in XP/XRT/XP cohort vs. 74.2% XP cohort (P=0.0862)]. However, an exploratory subgroup analysis of node-positive patients showed that 3-year DFS favored the chemoradiotherapy group (P=0.0365), suggesting that node-positive patients may benefit from adjuvant CRT attributed to better locoregional control. With a longer 7-year median follow-up, there remained no significant difference in DFS or OS for the entire intent-to-treat study population (DFS: HR 0.740, 95% CI: 0.520–1.050; P=0.0922; OS: HR 1.130, 95% CI: 0.775–1.647; P=0.5272) (11). Among the 396 patients with node-positive disease, there remained a significant difference in 3-year DFS (72% XP vs. 76% XPRT, P=0.04). Also among 163 patients with Lauren intestinal subtype histology, 3-year DFS also favored the addition of CRT (83% XP vs. 94% XPRT, P=0.01). The authors conclude that stratification of lymph node status and tumor histology are key factors in determining the benefit of adjuvant CRT after gastrectomy with D2 lymph node dissection.
After the positive outcome of SWOG/INT 0116, a follow-up randomized-controlled phase III trial conducted in the United States, Cancer and Leukemia Group B (CALGB) 80101, aimed to improve upon adjuvant fluoropyrimidine-based CRT. Between 2002–2009 CALBG 80101 randomly assigned 546 patients who had undergone complete resection of AJCC 6th edition stage IB through IV (M0) gastric or GEJ adenocarcinoma to epirubicin, cisplatin, and 5-flurouracil (ECF) or the INT0116 regimen (9). The adjuvant ECF cohort received epirubicin 50 mg/m2 and cisplatin 60 mg/m2 on day 1 with infusional 5-FU 200 mg/m2 per day continuously for 21 days, followed by chemoradiotherapy on day 28. Four weeks after radiotherapy, patients received 2 cycles of ECF. Radiotherapy was standardized at a total of 45 Gy delivered in 1.8 Gy daily fractions for 5 weeks with concurrent 5-FU 200 mg/m2 daily delivered as a protracted infusion. The authors reported a 5-year OS rate of 44% in the ECF cohort compared to 44% in the 5-FU/LV cohort (HR 0.98; 95% CI: 0.78–1.24, P=0.69). Five-year DFS rates were 39% in the 5-FU/LV cohort versus 37% in ECF cohort (HR 0.96; 95% CI: 0.77–1.20, P=0.94) (Table 1). Overall the addition of a more active systemic therapy (ECF) to postoperative CRT did not translate to improved outcomes. Some questions have been raised regarding possible suboptimal duration of chemotherapy used in this study (i.e., 3 as opposed to 6 cycles in perioperative chemotherapy trials). Furthermore, the pre-planned dose reduction of ECF in the experimental arm after patients completed CRT raises concern if proper dosing of an active chemotherapy agent may have been compromised. The relative contribution of epirubicin to anti-tumor activity remains debated especially in light of a recent neoadjuvant trial described later not reporting additional clinical benefit when it is added to a platinum and fluoropyrimidine backbone (12).
Adjuvant chemotherapy
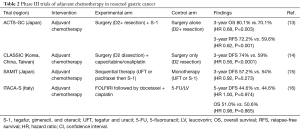
The Japanese Adjuvant Chemotherapy Trial of TS-1 in Gastric Cancer (ACTS-GC) was one of the first Asian multi-center, phase III trials to demonstrate clinical benefit of adjuvant chemotherapy after upfront surgery. This study randomly allocated 1,059 patients who had complete resection of Japanese Gastric Cancer Association classification stage II/IIIA/IIB gastric adenocarcinoma (II 50%, IIIA 30%, IIIB 10%) with D2+ dissection (94% D2, 6% D3) to the experimental arm of 1 year of adjuvant S-1 (tegafur, gimeracil, and oteracil 40 mg/m2 by mouth twice daily for four weeks of a six week cycle) or surgery alone (13). The authors reported a 3-year OS rate of 80.1% in the S-1 cohort versus 70.1% in the surgery-only cohort (HR 0.68; 95% CI: 0.52–0.87; P=0.003) (Table 2). The 3-year RFS rate was 72.2% in the S-1 group and 59.6% in the surgery-only group (HR 0.62; 95% CI: 0.50–0.77; P<0.001). At 5-year follow-up, the S-1 cohort continued to demonstrate robust results (17). The 5-year RFS was 65.4% in the S-1 cohort compared to 53.1% in the surgery-only cohort (HR 0.653; 95% CI: 0.537–0.793). This seminal phase III study established adjuvant chemotherapy as a standard in Asian patients with GC who underwent upfront gastrectomy with D2 lymph node dissection. An earlier phase I trial of S-1 and cisplatin in Western patients demonstrating tolerance of lower doses of S-1 in Caucasian relative to Asian patients along with the phase III FLAGS trial results limited the adoption of ACTS-GC outside of Asian countries (18).
Full table
The Capecitabine and Oxaliplatin Adjuvant Study in Stomach Cancer (CLASSIC) trial was a randomized-controlled, phase III trial conducted in South Korea, China, and Taiwan that enrolled 1,035 patients with complete resection of AJCC 6th edition stage II–IIIB GC and D2 dissection to evaluate the utility of adjuvant chemotherapy with XELOX (oxaliplatin 130 mg/m2 and capecitabine 1,000 mg/m2/dose twice daily for 14 days every three weeks for a total of 8 cycles) after surgery vs. surgery alone (14). Consistent with the ACTS-GC trial, the proportion of patients with tumors arising from the GEJ versus the stomach was low (3% vs. 97%). 3-year DFS rate was 74% in the XELOX arm compared to 59% in the surgery alone (HR 0.56; 95% CI: 0.44–0.72; P<0.0001) (Table 2). At a median of 5-year follow-up, the DFS rate was observed to be 68% in the experimental arm versus 53% in the control arm (HR 0.58; 95% CI: 0.47–0.72; P<0.0001) (19). Five-year OS rate was reported to be 78% in the adjuvant chemotherapy cohort versus 69% in the surgery only cohort (HR 0.66; 95% CI: 0.51–0.85; P=0.0015).
Results from the ACTS-GC and CLASSIC trials have resulted in the adoption of adjuvant chemotherapy as the preferred postoperative modality in Asian countries following gastrectomy and D2 lymph node dissection. The applicability of these results to Western populations, however, remains an open question given potential differences in disease biology and regional treatment patterns between the two populations. Compared to Western trials, studies based in Asia have exhibited a lower proportion of tumors arising from the gastroesophageal junction, and there are significant differences in rates of D1 compared to D2 dissection. The landmark Dutch D1 vs. D2 trial conducted in a Western patient population attempted to address the impact of differing surgical approaches alone as at the time of the study’s conduct, adjuvant therapy was not yet established as a standard of care. At initial reporting with 11 years of follow-up there was no significant difference in OS (D1 30% vs. D2 35%, P=0.53) (20). With longer follow-up 15-year OS still remains without a significant difference (D1 21% vs. D2 29%, P=0.34) (21). However, D2 dissection was associated with a lower rate of disease recurrence and incidence of gastric-cancer related deaths rates compared to D1 (HR 0.74; 95% CI: 0.59–0.93; P<0.01) though seemingly at a cost of higher postoperative mortality (D2 10% vs. D1 4%, P=0.004) and rates of surgical complications (D2 43% vs. D1 25%, P<0.0001). Follow up studies have established pancreas and spleen-sparing D2 approaches as oncologically equivalent with reported treatment-related mortality rates of <2–3% (22-24). As such, and with more updated surgical approaches, a D2 lymph node dissection is now usually recommended and carried out in high volume Western centers.
Efforts to investigate the utility of other chemotherapy agents in the adjuvant setting include the Stomach cancer Adjuvant Multi-Institutional group Trial (SAMIT) (Table 2). This was a large Japanese phase III clinical trial that enrolled patients with T4a or T4b gastric adenocarcinoma who had undergone D2 dissection to evaluate the survival outcomes of sequential therapy [paclitaxel followed by tegafur and uracil (UFT) or S-1] compared to monotherapy (UFT or S-1 alone) (15). A secondary randomization was performed to demonstrate the non-inferiority of UFT to S-1. UFT was previously a commonly administered agent in Japan until it became supplanted by S-1 following results from the ACTS-GC trial despite there not being a pre-existing direct comparison of the two. The primary endpoint was DFS with OS being one of the secondary endpoints. In total, 1,495 patients were randomly allocated equally to receive monotherapy of UFT alone (267 mg/m2 daily for 2 weeks followed by 1 week off), or S-1 alone (80 mg/m2 daily for 2 weeks followed by 1 week off), or sequential therapy of paclitaxel (80 mg/m2 weekly) followed by UFT or S-1. The monotherapy arms comprised of 48 weeks of planned therapy, while the sequential therapy arms lasted 49 weeks. The 3-year DFS rate for monotherapy was 54% vs. 57.2% for sequential therapy (HR 0.92; 95% CI: 0.80–1.07; P=0.273), thereby not demonstrating superiority of a sequential therapy approach. Three-year DFS rate for the UFT cohort was 53.0% compared to 58.2% in S-1 cohort (HR 0.81; 95% CI: 0.70–0.93; P=0.0048). As such UFT was inferior to S-1 validating the use of single agent S-1 as an adjuvant therapy approach.
The Intergroup Trial of Adjuvant Chemotherapy in Adenocarcinoma of the Stomach Trial (ITACA-S) was an Italian multicenter, randomized-controlled phase III clinical trial that randomly assigned 1,106 patients who had undergone curative gastrectomy to receive sequential therapy with FOLFIRI (irinotecan 180 mg/m2 on day 1 along with LV 100 mg/m2 with 5-FU 400 mg/m2 on days 1 and 2 followed by 600 mg/m2/day every 2 weeks for a total of four cycles) followed by docetaxel plus cisplatin (75 mg/m2 every three weeks for 3 cycles) compared to monotherapy with 5-FU/LV alone (De Gramont regimen) (16). The primary objective of the study was to assess whether adjuvant FOLFIRI followed by docetaxel and cisplatin conferred a survival benefit compared to 5-FU/LV. The authors reported 25% of patients undergoing a D1 lymphadenectomy, while 72% underwent a D2 lymph node dissection. In the 5-FU/LV cohort 450 of 520 patients (86.5%) successfully completed treatment compared to 421 of 552 patients (76.3%) in the sequential cohort. The resultant 5-year DFS rates were 44.6% in the experimental cohort compared to 44.6% in the control cohort (HR 1.00; 95% CI: 0.85–1.17; P=0.974). OS rates were also equivalent between the experimental and control arms, 51.0% vs. 50.6%, respectively (HR 0.98; 95% CI: 0.82–1.18; P=0.865). Thus, attempts to improve upon adjuvant therapy do not appear to be adequately addressed with escalation to multi-agent chemotherapy regimens.
Perioperative and neoadjuvant chemotherapy
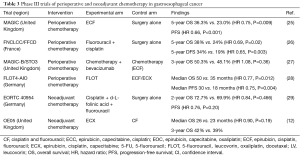
While adjuvant CRT dominated US practice patterns and likewise adjuvant chemotherapy in Asian countries, there remained interest in a perioperative approach theoretically improving surgical outcomes and addressing micrometastatic disease. The Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial was a phase III clinical trial that enrolled 503 patients deemed to have resectable clinical stage II+ gastric (74%), lower esophagus (15%), or GEJ adenocarcinoma (12%) and randomized to perioperative regimen (3 pre- and post-surgery cycles of ECF) (n=250) or surgery alone (n=253) (25). Of note 40% of the study population underwent a D2 lymphadenectomy with the remainder undergoing a lesser degree of lymph node dissection. The experimental arm demonstrated an improved 5-year OS rate of 36.3% compared to 23.0% in the control arm (HR 0.75; 95% CI: 0.60–0.93; P=0.009) along with improvement in progression-free survival (PFS) (HR 0.66; 95% CI: 0.53–0.81; P<0.001) (Table 3). Patients who underwent chemotherapy had a greater proportion pT1/2 tumors (51.7% vs. 36.8%; P=0.002) and pN0/1 (84.4% vs. 70.5%; P=0.01), indicative of pathologic down staging from neoadjuvant chemotherapy. Treatment adherence to chemotherapy was more favorable prior to surgery with 86% of patients in the experimental arm completing all 3 neoadjuvant cycles while 55% of patients were able to start their adjuvant cycles. Ultimately this translated to 42% of patients in the chemotherapy group completing all 6 planned cycles of ECF. The lower rates of completion in the MAGIC trial suggest tolerability of chemotherapy may be of concern, particularly post-operatively. Because this trial assessed the outcomes of perioperative treatment, it is unclear whether the improvement in survival can be attributed to only the neoadjuvant or the adjuvant therapy components.
Full table
Fédération Nationale des Centres de Lutte contre le Cancer/Fédération Francophone de Cancérologie Digestive (FNCLCC/FFCD) was a phase III French cooperative group clinical trial that randomly allocated 224 patients to receive perioperative fluorouracil plus cisplatin (2 or 3 cycles pre-surgery and 3 or 4 cycles post-surgery to comprise 6 cycles total of cisplatin 100 mg/m2 on day 1and fluorouracil 800 mg/m2 for 5 days every 28 days) compared to surgery alone (26). Despite being half the study size of the MAGIC trial, similar outcomes were reported with this 2-drug chemotherapy regimen with the experimental arm having a higher 5-year OS rate of 38% compared to 24% in the control arm (HR 0.69; 95% CI: 0.50–0.95; P=0.02). In addition, perioperative chemotherapy demonstrated improved 5-year DFS of 34% compared to 19% with surgery alone (HR 0.65; 95% CI: 0.48–0.89; P=0.003). Within the chemotherapy cohort, treatment adherence was more favorable pre-surgery with 87% of patients completing at least 2 neoadjuvant cycles while 48% of patients received at least one adjuvant cycle. Of note, only 23% of patients were able to complete all planned cycles of postoperative chemotherapy which the authors attributed to surgical complications and worsening nutritional status. Both the MAGIC and FNCLCC/FFCD trials established perioperative chemotherapy as an acceptable treatment approach to resectable gastric and GEJ cancers.
Modifications to standard perioperative chemotherapy regimens including incorporation of biologic agents have been investigated in an attempt to improve upon survival resectable gastric and gastroesophageal cancer. MAGIC-B/ST03 was a randomized-controlled, phase II–III clinical trial that randomly allocated 1,063 patients with resectable esophagogastric adenocarcinoma (lower esophageal 14%, GE junction 50%, gastric 36%) to chemotherapy alone (3 pre- and post-surgery 21-day cycles of ECX) or to chemotherapy plus the anti-angiogenic agent bevacizumab (7.5 mg/kg intravenously on day 1 of each 21-day cycle) (27). Patients in the experimental arm also received 6 further cycles of bevacizumab as a single agent after completing post-operative chemotherapy. Adherence to neoadjuvant therapy remained high with 89% of patients in the chemotherapy group and 88% of patients in the chemotherapy plus bevacizumab group receiving all 3 planned preoperative cycles. Consistent with earlier perioperative trials, adherence to adjuvant therapy demonstrated a drop-off with the control and experimental groups exhibiting 55% and 48%, respectively, starting their adjuvant cycles, while 40% and 37%, respectively, completed all 6 planned perioperative chemotherapy cycles. Disappointingly, 3-year OS was 50.3% in the control arm and 48.1% in the experimental arm (HR 1.08; 95% CI: 0.91–1.29; P=0.36). Furthermore, addition of bevacizumab did not appear to improve DFS (HR 1.04; 95% CI: 0.89–1.22; P=0.62) or PFS (HR 1.05; 95% CI: 0.89–1.23; P=0.56) (Table 3).
Recently, results from the FLOT4 trial suggest FLOT [docetaxel 50 mg/m2, oxaliplatin 85 mg/m2, LV 200 mg/m2, and 5-FU 2,600 mg/m2 (24-hour infusion) every 2 weeks] as a potential new standard in the perioperative treatment of resectable gastric or gastroesophageal junction cancer (28). FLOT4 was a randomized-controlled, German phase II–III clinical trial that randomly allocated 716 patients with clinically staged ≥ T2 and/or clinically N+ gastroesophageal adenocarcinoma to receive either FLOT (n=356) or anthracycline-based ECF (or ECX) (n=360) as established by the MAGIC trial. With a median of 43 months follow-up, FLOT demonstrated an improved median OS of 50 months compared to 35 months with ECF/ECX (HR 0.77; 95% CI: 0.63–0.94; P=0.012). The 3-year OS rate was 57% with FLOT compared to 48% with ECF/ECX. FLOT also improved PFS with a median PFS of 30 months compared to 18 months (HR 0.75; 95% CI: 0.62–0.91; P=0.004). In terms of toxicities, patients administered FLOT experienced more grade 3/4 neutropenia while patients on ECF/ECX experienced more grade 3/4 nausea and vomiting. In summary, this study demonstrated improved efficacy outcomes with perioperative FLOT compared to ECF/ECX, and should be considered as a regimen of choice for perioperative therapy if a triplet regimen is being considered.
With a common theme of treatment adherence being much higher in pre-operative versus post-operative phases of perioperative chemotherapy trials, questions have arisen as to whether efforts should be focused on neoadjuvant therapy alone. A purely neoadjuvant chemotherapy approach was explored in the German European Organization for Research and Treatment of Cancer (EORTC) 40954 trial (29). This phase III clinical trial randomly allocated 144 patients with clinically staged T3/4 gastric or GEJ adenocarcinoma to receive neoadjuvant chemotherapy (cisplatin 50 mg/m2 followed by d-L-folinic acid 500 mg/m2 and fluorouracil 2,000 mg/m2 on days 1, 8, 15, 22, 29, and 36 as part of two 48-day cycles) or surgery alone. The trial was initially powered to enroll 360 patients; however, enrollment was closed early due to poor accrual. The authors did not observe a survival benefit in the neoadjuvant arm with a 2-year OS rate of 72.7% vs. 69.9% with surgery alone (HR 0.84; 95% CI: 0.52–1.35; P=0.466) nor an improved PFS (HR 0.76; 95% CI: 0.49–1.16; P=0.20). Five patients (7.1%) were reported to have a complete pathologic response after neoadjuvant therapy. The authors concluded that the lack of a survival advantage with neoadjuvant chemotherapy was possibly due to an underpowered study and higher D2 resection rates (>90% in both arms vs. 40% in MAGIC) that may have masked the benefits of the experimental arm. To date, a purely neoadjuvant chemotherapy only approach remains difficult to recommend over perioperative chemotherapy with the preponderance of phase III trial results.
Further refinement of the choice of chemotherapy agents for resectable gastroesophageal cancer was delineated by the neoadjuvant United Kingdom Medical Research Council OE05 trial (12). This multi-center, randomized-controlled, phase III trial randomly allocated 897 patients with surgically resectable esophageal or Siewert types 1 and 2 GEJ adenocarcinoma to receive either 2 cycles of CF [two 3-weekly cycles of cisplatin (80 mg/m2 day 1) and fluorouracil (1 gm/m2 per day on days 1–4)] or 4 cycles of ECX [four 3-weekly cycles of epirubicin (50 mg/m2) and cisplatin (60 mg/m2) on day 1, and capecitabine (1,250 mg/m2) daily]. Median OS was reported as 23 months with CF compared to 26 months with ECX (HR 0.90; 95% CI: 0.77–1.05; P=0.19). Three-year OS rate was 39% with CF versus 42% with ECX. In short, intensification of neoadjuvant chemotherapy to 4 cycles of epirubicin, cisplatin, and capecitabine did not translate to a survival advantage and ultimately, this neoadjuvant approach could not be recommended as a standard of care. This trial, along with the results of the FLOT4 trial would argue against epirubicin being an agent of choice in 3-drug perioperative chemotherapy regimens.
Incorporation of CRT into perioperative chemotherapy
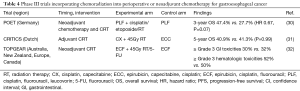
The potential for greater survival gains in resectable gastroesophageal cancer has been explored by combining benefits of perioperative chemotherapy (reducing systemic failure) and CRT (locoregional control) (Table 4). CRITICS is a phase III clinical trial that randomly allocated 788 patients with AJCC 6th edition stage Ib–IVa resectable GC to evaluate whether adjuvant chemoradiotherapy [45 Gy in 25 fractions together with cisplatin 20 mg/m2 (once weekly for 5 weeks) and capecitabine 575 mg/m2 (BID given on days receiving radiation)] after neoadjuvant chemotherapy and ≥ D1 dissection would result in improved OS compared to continuation of post-surgery chemotherapy [3 cycles of epirubicin 50 mg/m2 on day 1, cisplatin 60 mg/m2 on day 1, and capecitabine 1,000 mg/m2 BID from days 1–14 (ECC)] (31). Neoadjuvant chemotherapy administered to both arms consisted of 3 cycles of ECC. The authors reported a 5-year survival rate of 40.9% in the adjuvant chemoradiotherapy cohort compared to 41.3% in the postoperative chemotherapy cohort (P=0.99). Eighty-five percent of patients in chemotherapy arm compared to 81% of patients who received chemoradiotherapy completed pre-operative chemotherapy while 61% of patients vs. 63% started post-operative treatment. Within the chemotherapy arm, 47% of patients were able to complete treatment compared to 52% who received CRT. The authors concluded there was no improved OS with switching to adjuvant CRT versus continuing perioperative chemotherapy on an intent-to-treat basis.
Full table
A total neoadjuvant approach was studied in the German POET trial that investigated the role of adding neoadjuvant CRT to neoadjuvant chemotherapy in patients with adenocarcinomas purely of the gastroesophageal junction (30). POET was a multi-center, randomized-controlled, phase III clinical trial that randomly allocated 126 patients with locally advanced adenocarcinoma of the lower esophagus or gastric cardia to arm A [cisplatin 50 mg/m2 every 2 weeks, leucovorin 500 mg/m2 weekly, and 5-FU 2,000 mg/m2 weekly (PLF) every 6 weeks for 2.5 cycles followed by surgery] or arm B (2 cycles of PLF then cisplatin 50 mg/m2 days 1 and 8, etoposide 80 mg/m2 days 3–5, and radiation therapy 30 Gy in 15 fractions followed by surgery). The trial initially planned to enroll 354 patients; however, the study was terminated prematurely due to poor accrual. Seventy percent of patients in arm A underwent complete tumor resection vs. 72% in arm B. Higher pathologic complete response rate was observed in the CRT arm (15.6%) vs. the chemotherapy alone arm (2.0%) (P=0.03). Incorporation of preoperative CRT therapy demonstrated a numerically greater 3-year survival rate of 47.4% compared to 27.7% (HR 0.67; 95% CI: 0.41–1.07; P=0.07). Fourteen patients in arm A and 9 in arm B were observed to have local recurrence while 13 compared to 10 patients had distance recurrence. The lack of statistical significance for the survival benefit observed with the addition of CRT compared to neoadjuvant chemotherapy alone has been attributed to under accrual of the study.
Strategies to establish the benefit of neoadjuvant CRT in gastric adenocarcinomas regardless of primary tumor location include the ongoing Trial of Preoperative therapy for Gastric and Esophagogastric junction Adenocarcinoma (TOPGEAR). This is an ongoing randomized-controlled, phase III clinical trial that is planning to randomize up to 752 patients with surgically resectable disease to receive either perioperative ECF (3 cycles of pre-surgery ECF) or neoadjuvant CRT (2 cycles of pre-surgery ECF followed by 45 Gy RT/5-FU) to evaluate the safety and toxicity and efficacy of incorporating neoadjuvant CRT to perioperative chemotherapy. Both groups will receive 3 cycles of adjuvant ECF. Patients are being enrolled from centers in Australia, New Zealand, Europe, and Canada. An initial interim analysis was reported of the first 120 patients enrolled to demonstrate safety and feasibility of the approach (32). The authors found that 93% of patients in the ECF cohort were able to receive all cycles of preoperative chemotherapy compared to 98% of patients in CRT cohort. On the other hand, 65% vs. 53% of patients, respectively, completed postoperative cycles of chemotherapy with this difference not reported to be statistically significant. Grade ≥3 gastrointestinal and hematologic adverse events in the chemotherapy and CRT groups occurred in 32% vs. 30% and 50% vs. 52%, respectively. Surgical complications of anastomotic leak also appeared similar occurring in 3 patients (6%) in the chemotherapy group vs. 4 patients (8%) in the neoadjuvant chemoradiotherapy group. These interim results demonstrate the safety and feasibility of preoperative CRT in this setting and support ongoing enrollment to this trial. The higher treatment adherence to neoadjuvant therapy is promising and future efficacy results will be of great interest if this can validate a new treatment paradigm for resectable GC.
Future directions and incorporation of tumor molecular subtyping
As summarized, clinical outcomes have improved for non-metastatic esophagogastric cancer with the establishment of several multimodality approaches (2,6). In Asia, upfront gastrectomy and extended lymphadenectomy (D2) followed by adjuvant chemotherapy is standard practice. Perioperative chemotherapy represents a standard of care in Europe and parts of Australasia whereas, both perioperative chemotherapy and surgical resection with postoperative chemotherapy and CRT is routinely practiced in North America. Regardless of treatment approach, recurrence rates and OS remains suboptimal and further improvements are likely to be driven by improved biologic understanding to inform patient selection. The histologic classification of gastric adenocarcinomas has historically been based on the Lauren and World Health Organization classification systems which have limited ability to guide selection of specific therapies or serve as a predictive biomarker (2,3,33). While important, histopathologic classification alone does not reliably reflect biologic differences. Recent large-scale genomic and proteomic profiling of gastric adenocarcinomas by TCGA defined four major molecular subtypes of GC: microsatellite instability (MSI), Epstein-Barr virus (EBV)-associated, chromosomal instability (CIN), and genomically stable (GS) tumors (34). The esophageal cancer TCGA further added to our understanding and solidified strongly molecular similarities between GEJ adenocarcinoma and the CIN subtype of GC (35). The TCGA series have highlighted the differing frequencies of targetable genomic alterations among the molecular subtypes and the clinical applicability of molecular subtypes has been explored in a recent study incorporating data from TCGA to produce a relatively robust prediction model with prognostic and predictive value in resected GC (36). Using tumor gene expression profiling, the authors were able to generate RNA expression signatures that matched the four major TCGA molecular subtypes, and subsequently apply these signatures in patient datasets with a longer duration of clinical follow up than afforded by the GC TCGA analysis. The study authors observed the EBV subtype to be associated with the most favorable prognosis followed by the MSI and CIN subtypes (35). Of the four subtypes, the GS subtype carried the worst prognosis. Along with survival differences variation in therapeutic response to adjuvant chemotherapy differed by subtype, with the greatest benefit associated with the CIN subtype (HR 0.39; 95% CI: 0.16–0.94; P=0.03). Conversely, the least benefit appeared to be for patients with the GS subtype (HR 0.83; 95% CI: 0.36–1.89; P=0.65). Adjuvant chemotherapy was observed to provide moderate benefit to patients with the MSI subtype (HR 0.55; 95% CI: 0.22–1.3l; P=0.18). Finally, a scoring system derived from the tumor gene expression profiling was developed to provide a tool to further quantify the risk of recurrence [TCGA risk score raw = (1 − EBV probability) + (1 − MSI probability) + (GS probability ×2) + CIN probability]. Scores may range from 3.2 to 85.27 with the risk of recurrence defined as: low risk (<20), intermediate risk [20–30], and high risk of recurrence (>30). The TCGA recurrence score equation is derived on the basis that EBV and MSI are associated with the best prognosis and therefore the inverse of the probability of EBV and MSI is factored into the risk of recurrence. On the other hand, the GS gene expression value is doubled and additive to the risk score to reflect its contribution to poor prognosis. These results are very compelling in regards to moving beyond clinical variables alone and adding molecular subtyping to the predictive value of adjuvant therapy and prognosis for resected GC (35). However, further research is needed with validation in prospective studies and standardization of tumor gene expression tools that can be employed in the clinic.
While genomic level tumor profiling continues to be developed, currently validated molecular biomarkers may also already hold promise in resectable gastroesophageal cancer. Analogous to the implications of MSI status on benefit of adjuvant therapy in resected stage II colorectal cancers a recent post-hoc analysis of the MAGIC trial sought to evaluate the role of mismatch repair (MMR) deficiency (dMMR) and MSI among trial subjects (37). GCs with deficient DNA MMR harbor a high mutational burden leading to a higher propensity of harboring tumor neoantigens which may confer better anti-tumor immunosurveillance, and has translated into successful trial outcomes of immune checkpoint inhibitors in the metastatic setting (38). MSI was determined using validated DNA PCR-based methodologies, while MMR status was assayed with standard immunohistochemistry to ascertain expression or loss of MMR proteins. Of the 503 patients enrolled in the MAGIC trial, 303 were ultimately able to undergo successful determination of tumor MSI status. Tumors that were MSI-low comprised of 283 patients while 20 patients harbored MSI-high (MSI-H) tumors (6.6%). Interestingly, patients in the perioperative chemotherapy arm with either MSI-H or dMMR tumors had a median OS of 9.6 months compared with a median OS of 19.5 months in patients with neither MSI-H nor dMMR tumors (HR 2.18; 95% CI: 1.08–4.42; P=0.03). Patients in the surgery only cohort with MSI-H or dMMR tumors had a median OS that was not reached in comparison to a median OS of 20.5 months in patients without MSI-H nor dMMR tumors (HR 0.42; 95% CI: 0.15–1.15; P=0.09). These findings suggest that MSI or dMMR status may help identify patients who may or may not benefit from perioperative chemotherapy though this observation remains hypothesis-generating. This analysis also exemplifies the low proportion (6.6%) of MSI-H tumors in the non-metastatic setting making routine assessment difficult to recommend in current clinical practice. However, tumor MSI status may need to be accounted for in ongoing trials investigating the addition of PD-1 inhibitors among non-metastatic patients (Table 5).

Full table
Another recent study investigated the impact of tumor MSI on chemotherapy responsiveness and prognosis in patients with resectable gastroesophageal cancers as a post-hoc analysis of the data from the adjuvant CLASSIC trial (39). Similar to the MAGIC trial analysis, MSI status was determined by DNA PCR-based methodology. Of the 592 tumor samples able to be analyzed of the total 1,035 patients enrolled, 36 (6.1%) were MSI-H while the rest were microsatellite stable (MSS) (93.9%). MSI-H appeared to confer a favorable prognosis compared to MSS with an improved 5-year DFS rate amongst patients not treated with adjuvant therapy (HR 0.244; 95% CI: 0.069–0.867; P=0.0292). In addition, adjuvant chemotherapy versus surgery alone may strictly benefit DFS in patients with MSS tumors (HR 0.634; 95% CI: 0.485–0.828; P=0.0008) while no clear benefit can be established in the MSI-H cohort (HR 1.877; 95% CI: 0.284–12.390; P=0.5130). While the study results remain limited with the small patient numbers of MSI-H tumors, these findings increasingly support tumor MSI status as a guide to therapy and prognosis in resectable gastroesophageal cancers.
Conclusions
Although surgical resection of esophagogastric cancers is performed with curative intent in patients with locoregional disease, recurrence is common and carries a poor prognosis. Despite advances in multimodality treatment strategies in resectable GC, a global standard of care has not been achieved. While the FLOT4 trial has potentially practice-changing findings, uncertainties still exist as to the optimal timing and duration of chemotherapy, choice of chemotherapy agents, and benefit of radiation therapy (2,6). We await the results from several ongoing phase III trials investigating various perioperative treatment modalities to better address these issues. Molecular characterization efforts from clinically annotated datasets including the trials discussed have the promise to add biologic explanations as to heterogenous clinical outcomes (i.e., why two diffuse histology subtype patients with similar stage and management may have differing outcomes). Genomic features including MSI, tumor mutational burden, interferon and cytolytic signatures may aid in optimizing patient selection of immune-based approaches in the non-metastatic setting. Although a future of management algorithms being determined by molecular subtyping has yet to arrive, we anticipate more biomarker driven studies will enable this prospect to arrive soon and optimize outcomes in resectable esophagogastric cancer.
Acknowledgements
Funding: Efforts by J Chao in manuscript preparation were supported under National Institutes of Health Grant 5K12CA001727-23.
Footnote
Conflicts of Interest: J Chao has received research support (institutional), consulting, and speaker fees from Merck and consulting fees from Lilly. SJ Klempner has received speaker fees from Merck and consulting fees from Lilly and Boston Biomedical. The other authors have no conflicts of interest to declare.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Torre LA, Siegel RL, Ward EM, et al. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev 2016;25:16-27. [Crossref] [PubMed]
- Chan BA, Jang RW, Wong RK, et al. Improving Outcomes in Resectable Gastric Cancer: A Review of Current and Future Strategies. Oncology (Williston Park) 2016;30:635-45. [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-v49. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017;20:1-19. [Crossref] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Gastric Cancer, Version 5, 2017.
- Choi AH, Kim J, Chao J. Perioperative chemotherapy for resectable gastric cancer: MAGIC and beyond. World J Gastroenterol 2015;21:7343-8. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Lee J, Lim DH, Kim S, et al. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73. [Crossref] [PubMed]
- Fuchs CS, Niedzwiecki D, Mamon HJ, et al. Adjuvant Chemoradiotherapy With Epirubicin, Cisplatin, and Fluorouracil Compared With Adjuvant Chemoradiotherapy With Fluorouracil and Leucovorin After Curative Resection of Gastric Cancer: Results From CALGB 80101 (Alliance). J Clin Oncol 2017;35:3671-7. [Crossref] [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [Crossref] [PubMed]
- Park SH, Sohn TS, Lee J, et al. Phase III Trial to Compare Adjuvant Chemotherapy With Capecitabine and Cisplatin Versus Concurrent Chemoradiotherapy in Gastric Cancer: Final Report of the Adjuvant Chemoradiotherapy in Stomach Tumors Trial, Including Survival and Subset Analyses. J Clin Oncol 2015;33:3130-6. [Crossref] [PubMed]
- Alderson D, Cunningham D, Nankivell M, et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): an open-label, randomised phase 3 trial. Lancet Oncol 2017;18:1249-60. [Crossref] [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant Chemotherapy for Gastric Cancer with S-1, an Oral Fluoropyrimidine. N Engl J Med 2007;357:1810-20. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Tsuburaya A, Yoshida K, Kobayashi M, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol 2014;15:886-93. [Crossref] [PubMed]
- Bajetta E, Floriani I, Di Bartolomeo M, et al. Randomized trial on adjuvant treatment with FOLFIRI followed by docetaxel and cisplatin versus 5-fluorouracil and folinic acid for radically resected gastric cancer. Ann Oncol 2014;25:1373-8. [Crossref] [PubMed]
- Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93. [Crossref] [PubMed]
- Ajani JA, Faust J, Ikeda K, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol 2005;23:6957-65. [Crossref] [PubMed]
- Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:1389-96. [Crossref] [PubMed]
- Hartgrink HH, van de Velde CJH, Putter H, et al. Extended Lymph Node Dissection for Gastric Cancer: Who May Benefit? Final Results of the Randomized Dutch Gastric Cancer Group Trial. J Clin Oncol 2004;22:2069-77. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer 2004;90:1727-32. [Crossref] [PubMed]
- Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg 2006;93:559-63. [Crossref] [PubMed]
- Csendes A, Burdiles P, Rojas J, et al. A prospective randomized study comparing D2 total gastrectomy versus D2 total gastrectomy plus splenectomy in 187 patients with gastric carcinoma. Surgery 2002;131:401-7. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Cunningham D, Stenning SP, Smyth EC, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol 2017;18:357-70. [Crossref] [PubMed]
- Al-Batran SE, Pauligk C, Homann N, et al. LBA27_PR ‘Docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) for resectable esophagogastric cancer: updated results from multicenter, randomized phase 3 FLOT4-AIO trial (German Gastric Group at AIO) Ann Oncol 2017.28. [abstract].
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210-8. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Verheij M, Jansen EP, Cats A, et al. A multicenter randomized phase III trial of neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy in resectable gastric cancer: First results from the CRITICS study. J Clin Oncol 2016.34. Abstract 4000.
- Leong T, Smithers BM, Haustermans K, et al. TOPGEAR: A Randomized, Phase III Trial of Perioperative ECF Chemotherapy with or Without Preoperative Chemoradiation for Resectable Gastric Cancer: Interim Results from an International, Intergroup Trial of the AGITG, TROG, EORTC and CCTG. Ann Surg Oncol 2017;24:2252-8. [Crossref] [PubMed]
- Bosman FT, Carneiro F, Hruban RH, et al. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer, 2010.
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network, Analysis Working Group: Asan University, BC Cancer Agency, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Sohn BH, Hwang JE, Jang HJ, et al. Clinical Significance of Four Molecular Subtypes of Gastric Cancer Identified by The Cancer Genome Atlas Project. Clin Cancer Res 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Smyth EC, Wotherspoon A, Peckitt C, et al. Mismatch Repair Deficiency, Microsatellite Instability, and Survival: An Exploratory Analysis of the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) Trial. JAMA Oncol 2017;3:1197-203. [Crossref] [PubMed]
- Fuchs CS, Doi T, Jang RW, et al. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol 2017.35. Abstract 4003.
- Choi YY, Kim H, Yang HK, et al. Clinical impact of microsatellite instability in patients with stage II and III gastric cancer: Results from the CLASSIC trial. J Clin Oncol 2017.35. Abstract 4022.


