Gefitinib and celecoxib in advanced metastatic gastrointestinal tumors: a pilot feasibility study
Introduction
Gastrointestinal (GI) cancer (cancer of esophagus, stomach, intestines, liver, or pancreas) is a major health problem. Approximately 3.25 million people are diagnosed with the disease each year worldwide (1), with Brazil accounting for nearly 2% of these cases (1). The majority of GI tumors are epithelial in origin, and most patients present with advanced (regional or distant) disease (~60% patients for colorectal and esophageal cancer) with poor prognoses and low survival rates (2). Despite advances in surgery, radiotherapy, and chemotherapy, treatment for most patients is palliative. Indeed, the life expectancy for patients with advanced gastric cancer (with or without chemotherapy) is only 6 to 9 months (3). Chemotherapy for advanced GI cancer has some advantage over best supportive care (BSC), including improved quality of life; however, survival does not increase dramatically, with overall survival ranging from 6.0 to 12.0 months with chemotherapy vs. 2.5 to 5.0 months with BSC (4-9). For patients with advanced colorectal cancer with distant spread, 5-year survival is only 11% (10). There is a clear need for alternative treatment options that are effective in advanced GI cancers.
Increasing knowledge of the molecular events underlying carcinogenesis, tumor growth, and metastasis has provided new targets for therapy, including the epidermal growth factor receptor (EGFR) and cyclooxygenase-2 (COX-2). Elevated levels of EGFR and COX-2, both of which mediate events involved in tumorigenic processes, were observed in GI tumors (11-15). Increased EGFR expression was shown to correlate with more aggressive GI disease and poor survival in several studies (11,12,14-19). Similarly, COX-2 was found to be associated with poor prognosis and tumor recurrence in GI tumors (20-24). Indeed, COX-2 was also shown to promote angiogenesis and inhibit apoptosis in gastric tumor biopsies (25). As such, it was hypothesized that simultaneous inhibition of EGFR and COX-2 signaling pathways may be a novel treatment option capable of producing synergistic antitumor effects in patients with GI tumors.
Gefitinib (IRESSA®; AstraZeneca, Macclesfield, UK) is an orally active EGFR tyrosine kinase inhibitor. Phase I trials of gefitinib monotherapy demonstrated some activity in advanced GI cancer (26-29), with stable disease observed in several patients with colorectal and esophageal tumors. A phase II study also found that treatment with gefitinib (250 or 500 mg/day) was associated with disease control in 13/75 (18.3%) patients with metastatic gastric adenocarcinoma (30). In another phase II study, gefitinib (250 or 500 mg/day) was associated with a median progression-free and overall survival of 1.9 and 6.3 months, respectively, in patients with recurrent colorectal adenocarcinoma (31). Interestingly, an in vitro study conducted in human colon cancer cells showed that when gefitinib was combined with the COX-2 inhibitor SC-236, the two agents had a cooperative antiproliferative effect (32). This effect was accompanied by a reduction in the expression of COX-2 and angiogenic growth factors, such as vascular endothelial growth factor.
Celecoxib (Celebrex®; Pfizer Inc., New York, NY, USA) is a selective COX-2 inhibitor that has demonstrated potent suppression of colon polyps, which can lead to the development of colorectal cancer. However, enrollment in follow-up trials was inadequate and, as a result, regulatory requirements were not fulfilled and celecoxib was withdrawn in the USA and Europe as an adjunct to standard care in patients with familial adenomatous polyposis (33). The different mechanisms of action of gefitinib and celecoxib, together with in vitro evidence that suggests the two agents have a cooperative antiproliferative effect (30), provide a rationale for clinical evaluation of their combination. As such, we investigated the efficacy and tolerability of gefitinib in combination with celecoxib in patients with advanced or refractory GI tumors of epithelial origin.
Methods
Patient population
The study population consisted of adults (aged ≥18 years) with advanced or refractory, stage III/IV, histologically or cytologically confirmed GI tumors of epithelial origin (i.e., esophageal, gall bladder, colorectal, or pancreatic). Refractory patients had received previous treatment including ≥1 chemotherapeutic regimen with or without previous radiotherapy. However, patients with untreated advanced disease could participate if they were considered unsuitable for, or if they had refused, conventional chemotherapy. Patients with ≥1 measurable lesion according to Response Evaluation Criteria In Solid Tumors (RECIST), an Eastern Cooperative Oncology Group (ECOG) performance status of ≤3, and a life expectancy of >12 weeks were eligible.
Patients were ineligible to participate in the study in the event of: any evidence of severe or uncontrolled systemic disease (e.g., unstable or uncompensated respiratory, cardiac, hepatic, or renal disease); active duodenal or gastric ulcers; any other co-existing malignancy or malignancy diagnosed within the past two years (with the exception of basal cell carcinoma or cervical cancer in situ); unresolved chronic toxicity greater than Common Toxicity Criteria (CTC) grade 2 from prior therapies (except alopecia); evidence of incomplete healing from previous oncologic or other surgery, or any known hematologic bleeding dyscrasias; any contraindication to the use of celecoxib; pregnancy or breastfeeding. In addition, patients undergoing concomitant treatment with phenytoin, carbamazepine, barbiturates, rifampicin, or St John’s Wort were not eligible to participate. Furthermore, except for the study drugs, use of systemic treatments known to have an effect on GI tumors was not permitted during the trial. Radiotherapy, however, could be used outside the measurable lesions if necessary for symptomatic or healing purposes.
Patients were also excluded if any of the following laboratory parameters were recorded during screening: absolute neutrophil count <1.0×109/L; platelets 9/L; hemoglobin <9.0 g/dL; serum bilirubin >1.25 times the upper limit of normal (ULN); serum creatinine >1.8 mg/dL or creatinine clearance <60 mL/min; alanine aminotransferase or aspartate aminotransferase >2.5 times the ULN if no demonstrable liver disease, or >5.0 times the ULN in the presence of liver metastases.
Study design
This AstraZeneca-sponsored study (1839IL/0086) was a pilot, open-label, non-comparative, phase I/II study conducted at several centers in Brazil. The primary objective was to examine the safety and tolerability of the combination of gefitinib (250 mg/day) and celecoxib [400 mg twice daily (bid)] in advanced or refractory GI tumors. Secondary outcomes included the efficacy of the treatment regimen [objective response rate, disease control rate, progression-free survival (PFS), overall survival, duration of response] and the safety of combination therapy. An exploratory objective evaluated the association between tumor EGFR and COX-2 immuno-expression and tumor response. The trial was conducted in accordance with Good Clinical Practice and the ethical principles outlined in the revised Declaration of Helsinki. Local ethics committee approval was obtained before study initiation and all participants gave written, informed consent.
Eligible patients were administered gefitinib and celecoxib, both given orally, from day 1 until disease progression, unacceptable toxicity, or withdrawal. Wherever possible, patients were followed up for ≥6 months after the start of trial therapy, with assessment on day 15 and then every 28 days thereafter.
Safety and tolerability measures
The nature, incidence, and severity of adverse events (AEs) were recorded throughout the study. Routine hematology, biochemistry, and physical examinations were carried out during the seven days before study entry and during the treatment phase on day 1, day 15, and every 28 days thereafter. Urinalysis was performed as necessary. Both AEs and laboratory parameters were assessed using National Cancer Institute CTC version 2.0. Causality was assigned by the investigators.
In cases where toxicity was unacceptable, dose interruptions (≤14 days) were used as the first approach to manage toxicity. Repeat dose interruptions were permitted but if toxicity recurred on re-challenge and further interruptions were not considered to be sufficient to resolve toxicity, patients were either withdrawn from the study (for gefitinib-related toxicities) or underwent a dose reduction (for celecoxib-related toxicities). A single celecoxib dose reduction (from 400 to 200 mg bid) was permitted in patients experiencing recurring toxicity (> grade 2) to celecoxib. However, if serious GI toxicity was observed, celecoxib was discontinued and patients could continue on gefitinib monotherapy.
Efficacy measures
Objective tumor response (complete or partial response) was evaluated using RECIST within the 3 weeks prior to study entry, 6 weeks after the start of therapy, and every 12 weeks thereafter until disease progression. Patients were considered to have controlled disease if the RECIST criteria for complete response, partial response, or stable disease were at any time satisfied at or before trial closure. The duration of response was defined as the number of days from the first documented response until death/progression or the last on-study tumor assessment. Likewise, time to progression (TTP) was defined as the number of days from start of treatment on day 1 until disease progression/death or the last tumor assessment. Overall survival time was defined as the number of days from the first day of treatment until death or the last tumor assessment.
EGFR and COX-2 immunohistochemical assessment
Tumor EGFR and COX-2 immuno-expression was determined from biopsies taken at baseline (archived paraffin-embedded samples were permitted). Biopsy samples (≥2 mm2) underwent fixation in 4% neutral buffered formalin for 8 to 16 hours at room temperature followed by routine specimen dehydration using graded ethanols to xylene (or chloroform). Samples were then embedded longitudinally in paraffin under vacuum at 60 °C. In the event that paraffin-embedded tumor biopsies could not be provided, 5 μm thick sections were cut from tumor biopsies and applied to ten positively charged glass slides.
EGFR protein expression was assessed at the central laboratory by immunohistochemistry using the EGFR pharmDx kit (DAKO, Glostrup, Denmark), and a staining intensity of 0 to 3+. For the purpose of statistical analyses, staining intensities of 0 or 1+ were considered negative, and scores of 2+ or 3+ were considered positive for EGFR protein expression.
Immunohistochemistry for COX-2 was performed using a murine anti-COX-2 monoclonal antibody (clone 33, BD Transduction Laboratories, Lexington, KY, USA) at a dilution of 1:100. Samples were incubated for 16 hours at 4 °C, amplified using an avidin-biotin-peroxidase system, with antigen recovery performed under pressure (3.30 min) in sodium citrate solution (pH 6.0). The extension of stromal and tumoral COX-2 staining was assessed in a semiquantitative manner from 0 to 3+, where 0 and 1+ were considered negative and 2+ or 3+ were considered positive.
Statistical analysis
This was a pilot feasibility study and no formal statistical power calculations were performed. Nevertheless, a sample size of 30 patients was considered sufficient to examine the primary objective given that any event with an underlying incidence of 8% has a probability in excess of 90% of occurring in at least one patient out of 30.
The intent-to-treat population (i.e., all patients who enrolled and received study medication) was used to analyze efficacy parameters. Median duration of response, TTP, and overall survival were summarized using Kaplan-Meier methods along with the appropriate 95% confidence interval (CI). Tolerability outcomes were described using standard summary statistics.
Results
Patients
In total, 30 patients were enrolled into the study between December 2002 and April 2003 and their demographic characteristics are summarized in Table 1. Colorectal carcinoma was the most common primary GI tumor (83% of patients). Twenty-nine patients had received prior chemotherapy, with the majority receiving at least two previous regimens. Nearly one quarter of patients had also received prior radiotherapy. ECOG performance status was 0 to 1 in 90% of patients. All enrolled patients received at least one dose of gefitinib and celecoxib, and the median duration of treatment throughout the study was 70 days (range, 13 to 290 days).
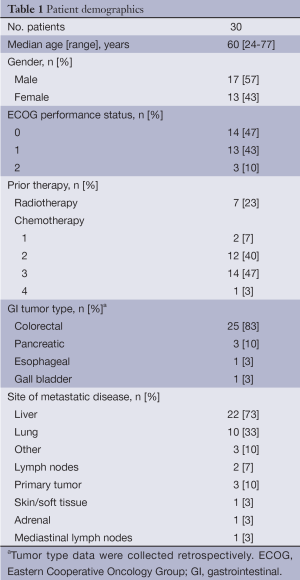
Full table
Treatment
Interruptions in gefitinib and celecoxib therapy were required in 17 (56.7%) and 17 (56.7%) patients, respectively. Only six patients (20.0%) required dose interruption because of toxicity related to gefitinib (diarrhea, acne, and erythema). Eleven patients (36.7%) required interruption in celecoxib therapy due to toxicity (hepatitis, vomiting, nausea, and gastric pain). Eleven patients required interruption in gefitinib therapy and six patients required interruption in celecoxib therapy for reasons other than toxicity, such as disease progression, surgery, and non-related toxicity. Five patients had their dose of celecoxib reduced (three cases due to toxicity, one case due to mental confusion, and one case due to patient misunderstanding of required dosing).
Safety and tolerability
In total, 28 patients (93%) experienced ≥1 AE during the study, most of which were mild to moderate in severity (Table 2). AEs were considered related to gefitinib in 20 (67%) patients and celecoxib in 11 (36.7%) patients. The most frequent AEs considered related to gefitinib were grade 1/2 acne and diarrhea. The most frequent AEs considered related to celecoxib were grade 1/2 stomatitis, nausea, diarrhea, and upper abdominal pain. Twelve patients (40%) experienced CTC grade 3/4 AEs (including fatigue, hepatitis, chest pain, pneumonia, perineal abscess, diarrhea, vomiting, hypertension, and abdominal pain). However, grade 3/4 AEs were considered by the investigator to be possibly related to gefitinib in only two patients; both grade 3 acne and folliculitis in one patient; and both grade 3 diarrhea and hypotension in one patient. One patient experienced grade 3 celecoxib-related hepatitis.
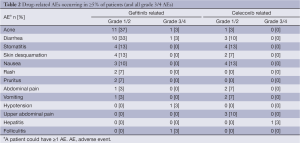
Full table
Of the three patients who required a reduction in the dose of celecoxib due to toxicity, one had a history of gastric sensitivity (dose was halved to 200 mg bid). No patients were withdrawn and there were no deaths due to AEs.
Efficacy
All 30 patients were included in the intent-to-treat population and were evaluable for efficacy. Twelve patients (40%) were classified as having stable disease during follow-up and 18 patients (60%) had progressive disease. The median TTP was 69 days (95% CI: 49-97) (Figure 1A).

Sixty percent of the patients (95% CI: 43-78) were alive at six months. The median overall survival time was 241 days; however, the 95% CI could not be estimated for this value due to censored data (Figure 1B).
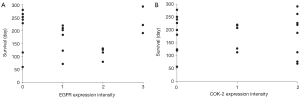
EGFR and COX-2 immuno-expression: relationship with tumor response
EGFR and COX-2 immuno-expression was evaluable for 20 and 21 patients, respectively. There was no significant association between either EGFR or COX-2 immuno-expression and TTP (data not shown) or overall survival (Figure 2).
Discussion
These data represent the only known clinical evaluation of gefitinib and celecoxib given in combination to patients with advanced/refractory GI cancer. While the results demonstrate that the regimen is feasible and well tolerated, disease control was only achieved in 12 patients (40%) who had confirmed stable disease for ≥8 weeks, and no patients were classified as complete or partial responders. In this study, an exploratory analysis failed to detect an association between either EGFR or COX-2 immuno-expression and TTP or survival.
In NSCLC, EGFR mutation has been shown to be a key predictive factor for the efficacy of gefitinib (34-36). To date, there is limited evidence on the role of activating EGFR mutations in determining response to gefitinib in colorectal cancer, and activating EGFR mutations are rare in colorectal cancer and do not seem to confer sensitivity to combination chemotherapy with gefitinib (37). Cetuximab, an anti-EGFR monoclonal antibody, is indicated for the treatment of EGFR-expressing metastatic colorectal cancer in combination with irinotecan; however, EGFR expression has been shown by some investigators to be unreliable and lack predictive value for survival in colorectal cancer (38,39). EGFR gene-copy number as determined by fluorescence in situ hybridization may be a potentially predictive tool for response rate and TTP with cetuximab (40,41), although some investigators failed to find a relationship between EGFR amplification and response rate, PFS, and overall survival with either cetuximab or gefitinib (42,43). Recent studies have indicated that the benefits of cetuximab in terms of response rates, PFS, and/or overall survival are limited to patients with wild-type K-Ras (44).
The celecoxib dose chosen for this study was 400 mg bid, a dose that had been previously recommended for patients with familial adenomatous polyposis based on data from a small study (n=77) that showed greater reductions in colorectal polyps (P=0.003) and polyp burden (P=0.001) compared with placebo over six months (45). In our study, three patients required a reduction in celecoxib dose to 200 mg bid for reasons of toxicity.
Since the completion of this study, rofecoxib and valdecoxib (the COX-2 inhibitors) were withdrawn from clinical use due to an apparent increased risk of serious thromboembolic AEs (including myocardial infarction and stroke) with long-term use compared with placebo (46). Two meta-analyses examined the cardiovascular risks of celecoxib and other non-steroidal anti-inflammatory drugs (NSAIDs) (47,48). The first analysis, which examined the incidence of cardiovascular events in randomized controlled studies of COX-2 inhibitors and traditional NSAIDs, found an increased risk of cardiovascular events with COX-2 inhibitors (47). However, the increased cardiovascular risk with celecoxib was observed only at doses ≥400 mg/day. The second analysis, which included observational rather than randomized studies, did not find an increased risk of cardiovascular events with celecoxib at doses commonly used in clinical practice (approximately 200 mg/day) (48). A more recent network meta-analysis indicated that celecoxib is associated with an increased risk of myocardial infarction and of cardiovascular death compared with placebo; however, the low event rates in the included trials meant that the estimates of rate ratios were imprecise, with wide credibility intervals, and statistical significance was not reached (49). A large study involving 20,000 patients with arthritis, either with or at risk of developing cardiovascular disease, is attempting to establish the true risk: benefit profile of celecoxib compared with traditional NSAIDs [Prospective Randomized Evaluation of Celecoxib Integrated Safety vs. Ibuprofen Or Naproxen (PRECISION); NCT00346216] (50). Recently, celecoxib has been withdrawn from use in familial adenomatous polyposis, in the USA and European markets, due to inadequate enrollment in follow-up clinical trials and concerns that any long-term benefits of treatment had not been shown to outweigh the increased risk of cardiovascular and GI side effects (33). Any further trials in this setting should therefore include careful follow-up of all patients, particularly if the 400 mg bid regimen is utilized, and interim toxicity and safety analyses should be integrated into the study design.
The combination of gefitinib and celecoxib used in this study was generally well tolerated. The most frequent AEs attributed to gefitinib were mild to moderate acne and diarrhea, while for celecoxib they were abdominal/upper abdominal pain, nausea, stomatitis, and diarrhea. These AEs were typical of each drug in terms of nature, incidence, and severity.
Although only limited activity was reported in this study, there have been other previous studies that have investigated the use of gefitinib in GI tumors. The combination of gefitinib (250 mg/day) and celecoxib (400 mg bid) has been evaluated in 15 chemonaïve patients with squamous-cell carcinoma (n=3) or adenocarcinoma (n=12) of the esophagus (51). Of the 14 patients who were evaluable for efficacy after two months, three patients (21%) had stable disease and remained in follow-up after a mean of 5.5 months (one patient had been lost to follow-up).
Gefitinib monotherapy (500 mg/day) has been evaluated in two phase II trials in patients with advanced esophageal cancer, with promising results. Response rates of 3% and 11% were reported, along with disease control rates of 31% and 37% (52,53). In both of these studies, the most common drug-related AEs were diarrhea [58% (52) and 59% (53)] and rash [47% (52) and 52% (53)].
Twenty-five (83%) of the 30 patients enrolled in the current study had colorectal cancer. Only limited data are available for gefitinib as a treatment for colorectal cancer, with several phase II studies of gefitinib in combination with standard treatment approaches (54,55). In the intent-to-treat population of one study of gefitinib in combination with capecitabine and oxaliplatin, three patients had a complete response, 14 had a partial response, and 11 had stable disease (55). Furthermore, in a phase II study of gefitinib in combination with the standard treatment option FOLFOX-4 in patients with advanced disease, 31 of 43 patients had a complete or partial response (54).
While studies in advanced NSCLC have found no difference in response rates between 250 and 500 mg/day doses of gefitinib (56,57), data from 75 patients with advanced GI cancers have indicated that the higher dose may be more effective, with disease control achieved in 13.9% and 22.9% of patients randomized to receive gefitinib 250 and 500 mg/day, respectively; median TTP was 0.9 and 1.6 months, respectively (30). While there were no statistically significant differences between the groups for either parameter, further investigations into the most appropriate dose for gefitinib to treat patients with advanced GI tumors are warranted.
In summary, this pilot, open-label, exploratory trial investigated the use of gefitinib plus celecoxib, a novel treatment combination, in patients with advanced GI tumors. The results of this study are encouraging for a population in whom care is generally palliative, and several other studies have shown promising activity with gefitinib in this setting. Nevertheless, there is still much to understand about the mode of action of EGFR and COX-2 inhibitors and how best to combine the agents with existing chemotherapeutic regimens. Moreover, the optimal dose for gefitinib in this setting remains undetermined and a definitive outcome regarding the long-term safety issues with COX-2 inhibitors is awaited.
Acknowledgements
We thank Fiona Boswell and Hannah FitzGibbon from Complete Medical Communications who provided editorial support funded by AstraZeneca.
Disclosure: No external funding was used to support this work. Editorial support for the preparation of this manuscript was funded by AstraZeneca. Iressa® is a trademark of the AstraZeneca group of companies. Celebrex® is a registered trademark of Pfizer, Inc.
References
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Updated 2010; cited 18 May 2011. Available online: http://globocan.iarc.fr
- Altekruse SF, Kosary CL, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975-2007, National Cancer Institute. Bethesda, MD. Available online: http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER web site, 2010.
- Hasham-Jiwa N, Kasakura Y, Ajani JA. Brief review of advances in the treatment of gastric carcinoma in North America and Europe, 1995-2001. Int J Clin Oncol 2002;7:219-24. [PubMed]
- Scheithauer W, Rosen H, Kornek GV, et al. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ 1993;306:752-5. [PubMed]
- Pyrhönen S, Kuitunen T, Nyandoto P, et al. Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995;71:587-91. [PubMed]
- Glimelius B, Ekström K, Hoffman K, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol 1997;8:163-8. [PubMed]
- Glimelius B, Hoffman K, Sjödén PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 1996;7:593-600. [PubMed]
- Meyerhardt JA, Fuchs CS. Chemotherapy options for gastric cancer. Semin Radiat Oncol 2002;12:176-86. [PubMed]
- Ajani JA. Evolving chemotherapy for advanced gastric cancer. Oncologist 2005;10 Suppl 3:49-58. [PubMed]
- American Cancer Society. Cancer facts and figures 2010. Atlanta, GA: American Cancer Society, 2010.
- Karameris A, Kanavaros P, Aninos D, et al. Expression of epidermal growth factor (EGF) and epidermal growth factor receptor (EGFR) in gastric and colorectal carcinomas. An immunohistological study of 63 cases. Pathol Res Pract 1993;189:133-7. [PubMed]
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer 2001;37 Suppl 4:S9-15. [PubMed]
- McKay JA, Murray LJ, Curran S, et al. Evaluation of the epidermal growth factor receptor (EGFR) in colorectal tumours and lymph node metastases. Eur J Cancer 2002;38:2258-64. [PubMed]
- Cohen RB. Epidermal growth factor receptor as a therapeutic target in colorectal cancer. Clin Colorectal Cancer 2003;2:246-51. [PubMed]
- García I, Vizoso F, Martín A, et al. Clinical significance of the epidermal growth factor receptor and HER2 receptor in resectable gastric cancer. Ann Surg Oncol 2003;10:234-41. [PubMed]
- Tokunaga A, Onda M, Okuda T, et al. Clinical significance of epidermal growth factor (EGF), EGF receptor, and c-erbB-2 in human gastric cancer. Cancer 1995;75:1418-25. [PubMed]
- Jonjić N, Kovac K, Krasević M, et al. Epidermal growth factor-receptor expression correlates with tumor cell proliferation and prognosis in gastric cancer. Anticancer Res 1997;17:3883-8. [PubMed]
- Kopp R, Rothbauer E, Ruge M, et al. Clinical implications of the EGF receptor/ligand system for tumor progression and survival in gastrointestinal carcinomas: evidence for new therapeutic options. Recent Results Cancer Res 2003;162:115-32. [PubMed]
- Gamboa-Dominguez A, Dominguez-Fonseca C, Quintanilla-Martinez L, et al. Epidermal growth factor receptor expression correlates with poor survival in gastric adenocarcinoma from Mexican patients: a multivariate analysis using a standardized immunohistochemical detection system. Mod Pathol 2004;17:579-87. [PubMed]
- Sheehan KM, Sheahan K, O’Donoghue DP, et al. The relationship between cyclooxygenase-2 expression and colorectal cancer. JAMA 1999;282:1254-7. [PubMed]
- Lim HY, Joo HJ, Choi JH, et al. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res 2000;6:519-25. [PubMed]
- Shamma A, Yamamoto H, Doki Y, et al. Up-regulation of cyclooxygenase-2 in squamous carcinogenesis of the esophagus. Clin Cancer Res 2000;6:1229-38. [PubMed]
- Tomozawa S, Tsuno NH, Sunami E, et al. Cyclooxygenase-2 overexpression correlates with tumour recurrence, especially haematogenous metastasis, of colorectal cancer. Br J Cancer 2000;83:324-8. [PubMed]
- Shi H, Xu JM, Hu NZ, et al. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol 2003;9:1421-6. [PubMed]
- Tatsuguchi A, Matsui K, Shinji Y, et al. Cyclooxygenase-2 expression correlates with angiogenesis and apoptosis in gastric cancer tissue. Hum Pathol 2004;35:488-95. [PubMed]
- Baselga J, Rischin D, Ranson M, et al. Phase I safety, pharmacokinetic, and pharmacodynamic trial of ZD1839, a selective oral epidermal growth factor receptor tyrosine kinase inhibitor, in patients with five selected solid tumor types. J Clin Oncol 2002;20:4292-302. [PubMed]
- Herbst RS, Maddox AM, Rothenberg ML, et al. Selective oral epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 is generally well-tolerated and has activity in non-small-cell lung cancer and other solid tumors: results of a phase I trial. J Clin Oncol 2002;20:3815-25. [PubMed]
- Ranson M, Hammond LA, Ferry D, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol 2002;20:2240-50. [PubMed]
- Nakagawa K, Tamura T, Negoro S, et al. Phase I pharmacokinetic trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (‘Iressa’, ZD1839) in Japanese patients with solid malignant tumors. Ann Oncol 2003;14:922-30. [PubMed]
- Doi T, Koizumi W, Siena S, et al. Efficacy, tolerability, and pharmacokinetics of gefitinib (‘Iressa’, ZD1839) in pretreated patients with metastatic gastric cancer [poster]. Poster 1036 presented at the ASCO, Chicago, IL, 2003.
- Rothenberg ML, LaFleur B, Levy DE, et al. Randomized phase II trial of the clinical and biological effects of two dose levels of gefitinib in patients with recurrent colorectal adenocarcinoma. J Clin Oncol 2005;23:9265-74. [PubMed]
- Tortora G, Caputo R, Damiano V, et al. Combination of a selective cyclooxygenase-2 inhibitor with epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 and protein kinase A antisense causes cooperative antitumor and antiangiogenic effect. Clin Cancer Res 2003;9:1566-72. [PubMed]
- European Medicines Agency. European Medicines Agency concludes on use of celecoxib in familial adenomatous polyposis [Internet]. Updated 2011; cited 8 November 2011. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/public_health_alerts/2011/05/human_pha_detail_000029.jsp&mid=WC0b01ac058001d126&jsenabled=true
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Ogino S, Meyerhardt JA, Cantor M, et al. Molecular alterations in tumors and response to combination chemotherapy with gefitinib for advanced colorectal cancer. Clin Cancer Res 2005;11:6650-6. [PubMed]
- Doger FK, Meteoglu I, Tuncyurek P, et al. Does the EGFR and VEGF expression predict the prognosis in colon cancer? Eur Surg Res 2006;38:540-4. [PubMed]
- Kountourakis P, Pavlakis K, Psyrri A, et al. Clinicopathologic significance of EGFR and Her-2/neu in colorectal adenocarcinomas. Cancer J 2006;12:229-36. [PubMed]
- Hamilton SR. Targeted therapy of cancer: new roles for pathologists in colorectal cancer. Mod Pathol 2008;21 Suppl 2:S23-30. [PubMed]
- Cappuzzo F, Finocchiaro G, Rossi E, et al. EGFR FISH assay predicts for response to cetuximab in chemotherapy refractory colorectal cancer patients. Ann Oncol 2008;19:717-23. [PubMed]
- Cascinu S, Berardi R, Salvagni S, et al. A combination of gefitinib and FOLFOX-4 as first-line treatment in advanced colorectal cancer patients. A GISCAD multicentre phase II study including a biological analysis of EGFR overexpression, amplification and NF-kB activation. Br J Cancer 2008;98:71-6. [PubMed]
- Italiano A, Follana P, Caroli FX, et al. Cetuximab shows activity in colorectal cancer patients with tumors for which FISH analysis does not detect an increase in EGFR gene copy number. Ann Surg Oncol 2008;15:649-54. [PubMed]
- Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 2009;27:2091-6. [PubMed]
- Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000;342:1946-52. [PubMed]
- Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med 2004;351:1709-11. [PubMed]
- Kearney PM, Baigent C, Godwin J, et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 2006;332:1302-8. [PubMed]
- McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 2006;296:1633-44. [PubMed]
- Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ 2011;342:c7086. [PubMed]
- Pfizer Inc. Prospective Randomized Evaluation of Celecoxib Integrated Safety vs Ibuprofen or Naproxen (PRECISION) [study registered on Clinicaltrials.gov] [Internet]. Updated 2011; cited 25 March 2011. Available online: http://clinicaltrials.gov/ct2/show/NCT00346216?term=nct00346216&rank=1
- Vervenne WL, Bollen JM, Bergman JJGHM, et al. Evaluation of the antitumor activity of gefitinib (ZD1839) in combination with celecoxib in patients with advanced esophageal cancer. J Clin Oncol 2004;22:abstr 4054.
- Janmaat ML, Gallegos-Ruiz MI, Rodriguez JA, et al. Predictive factors for outcome in a phase II study of gefitinib in second-line treatment of advanced esophageal cancer patients. J Clin Oncol 2006;24:1612-9. [PubMed]
- Ferry DR, Anderson M, Beddard K, et al. A phase II study of gefitinib monotherapy in advanced esophageal adenocarcinoma: evidence of gene expression, cellular, and clinical response. Clin Cancer Res 2007;13:5869-75. [PubMed]
- Fisher GA, Kuo T, Ramsey M, et al. A phase II study of gefitinib, 5-fluorouracil, leucovorin, and oxaliplatin in previously untreated patients with metastatic colorectal cancer. Clin Cancer Res 2008;14:7074-9. [PubMed]
- Gelibter AJ, Gamucci T, Pollera CF, et al. A phase II trial of gefitinib in combination with capecitabine and oxaliplatin as first-line chemotherapy in patients with advanced colorectal cancer. Curr Med Res Opin 2007;23:2117-23. [PubMed]
- Fukuoka M, Yano S, Giaccone G, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial) J Clin Oncol 2003;21:2237-46. [PubMed]
- Kris MG, Natale RB, Herbst RS, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 2003;290:2149-58. [PubMed]


