Durable response with a combination of imatinib and sorafenib in KIT exon 17 mutant gastrointestinal stromal tumor
Case report
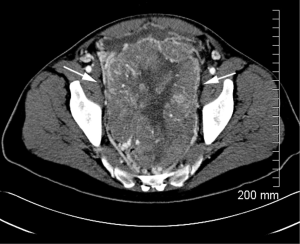
A 54-year-old white male presented to his primary physician for routine examination. He was found to have a persistently increasing PSA (>20) and he subsequently underwent a prostate biopsy. Pathology was reported as CD117 positive (CD34, S100, smooth muscle actin and keratin negative) spindle cell neoplasm consistent with a gastrointestinal stromal tumor (GIST). There were 10 mitoses per 50 HPF and subsequent gene sequence analysis demonstrated N822K mutation at c-kit exon 17. Staging CT scan of his abdomen and pelvis demonstrated a 13 cm × 6 cm lesion extending down from his rectum to the level of the prostate as well as a 3 cm hepatic lesion concerning for metastatic disease (Figure 1). Treatment was initiated with imatinib 400 mg daily with follow-up CT scans every 3-4 months.
Six months after commencement of imatinib, CT scan showed interval increase in the size of the pelvic mass to 19 cm × 9 cm as well as several enlarged mesenteric lymph nodes and peritoneal metastases. Imatinib was increased to 800 mg daily. Additionally, he developed multiple right lower extremity deep venous thrombosis (DVTs) and started anticoagulation therapy with warfarin. Repeat CT scan three months later showed necrosis within multiple tumors, however the patient developed a new 3.2 cm × 2.3 cm lesion consistent with progression of disease. Imatinib was stopped and the patient was started on sunitinib 50 mg four weeks on and two weeks off.
While on sunitinib, he developed significant anemia with hemoglobin of 4.9 requiring admission to the hospital and multiple transfusions. Work-up revealed Coombs positive autoimmune hemolytic anemia managed with steroids. Additionally he developed new bilateral lower extremity DVTs while on coumadin and an IVC filter was placed. CT scan during that admission showed progression of disease.
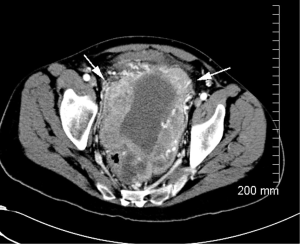
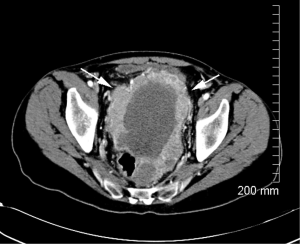
Sunitinib was stopped and he began treatment with sorafenib 400 mg twice daily. CT scans after three months of treatment showed marked decrease in size of the primary tumor (Figure 2), but follow-up CT scans after six months on sorafenib revealed a new soft tissue mass in the left lower abdomen, as well as enlargement and necrosis of multiple soft tissue masses along the right paracolic gutter. There was also decrease in two masses in the right lower quadrant. At that time imatinib, 400 mg every other day was added to sorafenib 400 mg twice daily. Follow-up CT scans showed stable disease for almost one year after which he developed numerous peritoneal lesions (Figure 3). Imatinib was increased to 400 mg daily and surveillance CT scans have since remained stable over the last one year using combination treatment of imatinib and sorafenib.
Discussion
While a relatively rare gastrointestinal malignancy, GISTs are the most common primary mesenchymal tumor arising in the GI tract. Eighty five to ninety percent of all GISTs arise in the stomach and small intestine and approximately 4% arise in the rectum (1).
This group of tumors is believed to be derived from the interstitial cells of Cajal, which are responsible for coordinating peristaltic contractions throughout the GI tract. Studies have demonstrated that these cells commonly express KIT tyrosine kinase (CD117). Sixty eight percent of mutations to KIT occur in the juxtamembrane portion (exon 11) while only 1% are believed to occur in exon 17 (2).
Surgical resection remains the only potential curative treatment of GIST. However, recurrence rates following surgical resection have been reported from 40-90% (3).
Understanding of the molecular oncogenesis of GIST has prompted investigations in the use of targeted therapy to block the function of this tyrosine kinase. The first of these medications, imatinib produced significant responses with median progression free survival in the US S0033 phase 3 trial of 18 months and median overall survival of 55 months (4). Additionally, there was no significant difference in outcome between doses of imatinib 400 mg daily and 800 mg daily except in exon 9 mutated patients where the estimated risk of progression or death was reduced by 42% in the high dose arm compared to the lower (5). Twelve to fourteen percent of GIST patients have primary resistance to imatinib while 40-50% develop secondary resistance with progression of disease within 2-3 years (6,7). Resistance to tyrosine kinase inhibitors is of special consideration in exon 17 mutations in both the primary and secondary settings (8). Imatinib has been demonstrated to be more effective in juxtamembrane mutations like KIT exon 11 and PDGFR exon 12 and less effective in those mutations affecting activation loops like KIT exon 17 and PDGFR exon 18 (8).
Exon 17 mutants have also been shown to develop cross-resistance to sunitinib. Sunitinib has been approved as second line treatment following development of resistance or treatment failure with imatinib (9). A 2012 retrospective analysis of sorafenib as third or fourth line therapy in advanced GIST demonstrated a median overall survival of 13.5 months (10). Sorafenib, with its antagonism of the activation loop in exon 17 mutants, has provided rationale for its use in imatinib-resistant patients (11). Studies have suggested a role for intermittent imatinib in exon 17 mutant GIST (12). Liegl et al. reported on the heterogeneity of kinase inhibitors resistance mechanism in GIST, in a study of 53 GIST metastases in 14 patients, 6 out of 14 patients had two to five different secondary mutations in separate metastases. Furthermore, three patients were found to have two secondary KIT mutations within the same metastasis thus potentially raising the question of a consideration for studies evaluating combining TKI monotherapies if deemed tolerable and beneficial (13). While our patient developed resistance to imatinib six months after initiating therapy, he has had quite durable responses to sorafenib plus imatinib lasting more than two years. Recently, regorafenib has been approved for 3rd line treatment of GIST following progression after imatinib and sunitinib. GRID—a randomized phase 3 trial of 133 patients treated with regorafenib 160 mg once daily three out of four weeks showed a significantly improved PFS of 4.8 versus 0.9 months in the placebo arm (n=66) (14). Further studies are warranted to understand the role of regorafenib in patients with exon 17 mutations.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Zhao X, Yue C. Gastrointestinal stromal tumor. J Gastrointest Oncol 2012;3:189-208. [PubMed]
- Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol 2008;3:557-86. [PubMed]
- Rossi CR, Mocellin S, Mencarelli R, et al. Gastrointestinal stromal tumors: from a surgical to a molecular approach. Int J Cancer 2003;107:171-6. [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [PubMed]
- Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53. [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [PubMed]
- Van Glabbeke M, Verweij J, Casali PG, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: a European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol 2005;23:5795-804. [PubMed]
- Wozniak A, Floris G, Debiec-Rychter M, et al. Implications of mutational analysis for the management of patients with gastrointestinal stromal tumors and the application of targeted therapies. Cancer Invest 2010;28:839-48. [PubMed]
- Gajiwala KS, Wu JC, Christensen J, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci U S A 2009;106:1542-7. [PubMed]
- Montemurro M, Gelderblom H, Bitz U, et al. Sorafenib as third- or fourth-line treatment of advanced gastrointestinal stromal tumour and pretreatment including both imatinib and sunitinib, and nilotinib: a retrospective analysis. Eur J Cancer 2013;49:1027-31. [PubMed]
- Guo T, Agaram NP, Wong GC, et al. Sorafenib inhibits the imatinib-resistant KITT670I gatekeeper mutation in gastrointestinal stromal tumor. Clin Cancer Res 2007;13:4874-81. [PubMed]
- Revheim ME, Kristian A, Malinen E, et al. Intermittent and continuous imatinib in a human GIST xenograft model carrying KIT exon 17 resistance mutation D816H. Acta Oncol 2013;52:776-82. [PubMed]
- Liegl B, Kepten I, Le C, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol 2008;216:64-74. [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [PubMed]