Adjuvant radiation provides survival benefit for resected pancreatic adenocarcinomas of the tail
Introduction
Pancreatic cancer has one of the highest mortality to incidence ratios of all cancers, with one of the smallest clinical windows of intervention (1). By location, pancreatic cancers are found in the head, the uncinate, the body, and/or the tail, yet no data are available on whether location influences genomic characteristics of the individual tumor profile. Tumors located in the pancreatic tail are usually diagnosed incidentally, as they do not present with the “painless jaundice” seen in pancreatic head tumors (2). Their delayed presentation means that, if discovered, they are usually diagnosed as later stage cancers than the much more common tumors located in the pancreatic head. A recent SEER analysis suggested that in localized, resectable disease (per AJCC 8th edition staging), clinical outcomes are not significantly different based on tumor location (3). However, a National Cancer Database analysis reported a significantly longer 5-year overall survival (OS) for tumors located in the tail (32% vs. 11%) in stage I pancreatic cancers (4). Over the past thirty years, there has been little progress in the high rates of distant failure in patients with pancreatic cancer. Ultimately, treatment strategies including surgical resection with negative margins provide the only chance for cure. The resected 5-year survival remains poor, with rates still in the 20–24% range overall, and subgroups of patients with node and margin negative resection up to 39% (5).
Adjuvant therapy strategies for pancreatic cancer remain controversial. In the era prior to gemcitabine chemotherapy, the GI Tumor Study Group (GITSG) completed a small randomized trial comparing pancreatic cancer surgery alone to surgery combined with adjuvant 5-FU based chemoradiation that was delivered with split course technique (6). The adjuvant arm had an improved median survival (20 vs. 11 months, P=0.035) and subsequent patients who were not randomized but were treated on the CRT arm also had median OS of 18 months supporting the seeming superiority of CRT (7). Over the years, subsequent European trials have attempted to confirm these findings but have been unable to do so (8), with results from the ESPAC-1 trial showing that CRT was inferior to chemotherapy alone (9). These results have been criticized by other investigators for inadequate radiation techniques (lack of quality assurance, split course regimens, non-standard RT dose) as well as having only 53% of the patients enrolled included in the final data analysis (10). Nonetheless, based on the superiority of chemotherapy alone reported in the European trials, the Charite Onkologie Clinical (CONKO-001) trial (11) subsequently compared gemcitabine as single agent with observation and reported superior outcomes with improved median survival of 22.8 vs. 20.2 months (P=0.005).
While CRT has been significantly displaced in Europe in favor of systemic gemcitabine chemotherapy alone as the standard adjuvant therapy recommendation, the role of radiation therapy (RT) has received further study in the U.S. The Radiation Therapy and Oncology Group (RTOG) 9704 study incorporated a continuous regimen of 50.4 Gy delivered in 28 daily fractions with 5-FU preceded by a randomization for either one cycle of gemcitabine or 5-FU. Following CRT, the same chemotherapy was delivered for an additional 3 months (12). In the updated 5-year analysis, Regine et al. reported a trend on multivariate analysis (MVA) for improved OS with the gemcitabine arm with a 5-year OS of 22% compared with 18% (13). Results from this trial showed that the site of first failure in the majority (73%) of patients was distant. Further analysis from this study indicated that positive lymph nodes (14) and postoperative CA 19-9 values ≥90 U/mL were associated with worse survival (15). The RTOG investigators also evaluated their outcomes with respect to adherence to protocol guidelines, reporting that patients who were not treated according to protocol had worse median survival (10). In addition, the outcomes of those patients were compared with the outcomes of patients with pancreatic head tumors on RTOG 9704 treated with gemcitabine who also had a post-operative CA 19-9 <90 U/mL. Median survivals were consistent with those reported in the CONKO-001 trial (16).
Recent molecular data suggests that biomarkers may be the key to differentiating tumors with a local vs. distant pattern of failure (17). Studies from rapid autopsy series have shown that 30% of patients die with locally destructive tumors compared to 70% with disseminated metastasis and that patients with intact SMAD4 (DPC4) on immunohistochemistry were more likely to die of local disease (18). These findings suggest that there may indeed be subsets of patients with pancreatic cancer who may benefit from adjuvant RT. Our institution previously reported (19) on the potential significance of the radiation sensitivity index (RSI), evaluated the effect of radiosensitive patients with adverse prognostic factors who received RT, and found an improved median survival (31.2 vs. 13.2 months, P=0.04) when compared with patients with radioresistant tumors (20).
Few data exist specifically evaluating outcomes of subsets of patients with pancreatic tail cancer. Whether tumor location in pancreatic cancers is a manifestation of variant biological behavior and whether patients should be risk stratified by such a categorization remains to be seen. Current National Comprehensive Cancer Network (NCCN) guidelines recommend either a clinical trial, chemotherapy alone, or a regimen including chemoradiation for adjuvant therapy in pancreatic cancers, regardless of location (21). We thus sought to evaluate our institutional experience with adjuvant RT in surgically resected pancreatic tail cancers.
Methods
Patient selection
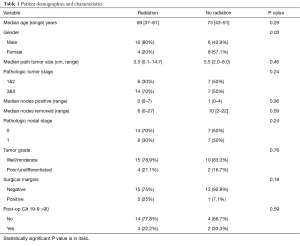
After obtaining IRB approval, a retrospective review identified 34 patients with pancreatic cancers located in the tail that underwent distal pancreatectomy between 2002–2012 at Moffitt Cancer Center. Patients were treated per our institution’s pancreatic cancer clinical pathway, which included the initial staging studies of endoscopic ultrasound (EUS), pancreas protocol computer tomography (CT), and Positron Emission Tomography (PET)/CT scan. The entire cohort was followed for a median duration of 18.2 months. Only those determined to have locally confined, non-metastatic disease amenable to distal pancreatectomy were included in this study. Additionally, patients with tumors located in the head or body of the pancreas, or underwent total pancreatectomy were also excluded. Patient characteristics are summarized in Table 1.
Full table
Statistical analysis
Patient characteristics were analyzed between patients treated with and without adjuvant RT with Pearson chi-square tests. The cutoff for CA 19-9 was set based on the findings from RTOG 97-04 (15). OS was evaluated using Kaplan-Meier survival functions. Significance was evaluated with Mantel-Cox log ranks. Significant predictors of survival functions were then further analyzed using multivariate Cox regression. For multiple variables that were significant, multivariate regression was performed. Statistical significance was set at P<0.05. All statistical analysis and figure artwork was performed and generated using SPSS v 23.0.0.2 (IBM, Armonk, NY, USA).
Results
Patient and tumor characteristics
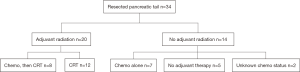
After undergoing distal pancreatectomy, 20 patients received adjuvant radiation while 14 patients did not. Irradiated patients were treated to a median dose of 50.4 Gy in 28 fractions with concurrent 5-FU (fluorouracil) chemotherapy as the complete regimen in 12/20 (60%) and in addition to systemic gemcitabine therapy in 8/20 (40%). Of the 14 that did not receive adjuvant RT, 7 patients received chemotherapy alone with gemcitabine, 5 did not receive any adjuvant therapy and the chemotherapy status of 2 patients was unknown (Figure 1).
Patient demographics were well matched between the two groups except for gender. Notably, the majority of the patients receiving radiation had tumors with a post-operative CA 19-9 that was <90 and that were well to moderately differentiated with negative margins and nodes. Patients who received adjuvant radiation were much more likely to be male (80% vs. 42.9%, P=0.03). However, subsequent sub-group analysis for gender was not a confounder for survival outcomes. Otherwise, pathologic and surgical features were equally distributed between the two groups. Specifically, pathologic tumor size, stage, number of positive nodes, number of nodes removed, surgical margins, and post-operative CA 19-9 were not significantly different (Table 1).
OS
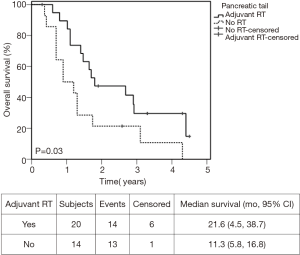
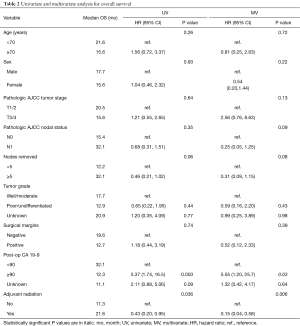
Univariate analysis (UVA) identified adjuvant radiation (HR =0.43, P=0.036) and post-operative CA 19-9 ≥90 (HR =5.37, P=0.003) as statistically significant predictors for survival (Table 2). In addition, removing ≥5 lymph nodes approached significance (HR =0.46, P=0.06). Therefore, all three variables were included in multivariate cox-regression analysis. However, only post-operative CA 19-9 <90 (HR =0.18, P=0.03) and adjuvant radiation (HR =0.15, P=0.006) remained statistically significant independent predictors for survival. Median survival in patients who received adjuvant radiation was 21.6 months, compared to 11.3 months in those who did not (Figure 2, P=0.03). Two-year OS were 47% and 21%, respectively.
Full table
Discussion
This study has shown that adjuvant RT containing regimens were associated with improved survival for pancreatic tail tumors. In our analysis, the majority of patients receiving adjuvant CRT regimens had a postoperative CA 19-9 <90 U/mL and underwent R0 resection for node negative tail tumors that were pathologic stage T3/4. The median survival of 21.6 months is concordant with the gemcitabine arm of the CONKO-001 trial and the RTOG 9704 analysis of pancreatic head tumors with CA 19-9 <90 U/mL that were treated according to protocol guidelines. In addition, 25% of the patients in the irradiated group in the present analysis had tumors resected with positive surgical margins, suggesting that adjuvant CRT may have obviated the adverse prognostic significance of R1 resection for these tail primary tumors.
The optimal adjuvant therapy regimen for resected pancreatic cancers remains controversial. GITSG 9731 provided some of the first prospective evidence that adjuvant chemoradiation improves survival after margin negative resection (6). However, in other clinical trials, the survival benefit of adjuvant radiation has been controversial, as seen in ESPAC-1 and EORTC 40013 (9,22). Criticisms for those early clinical trials include the anachronistic split dose fractionation scheme and the median 40 Gy delivered was likely an ineffectually low dose. Currently, NCCN guidelines still recommend adjuvant radiation as an acceptable means of management as level 2A evidence (21).
Current evidence is sparse with respect to adjuvant therapy outcomes specific to non-pancreatic head tumor locations. Ruess et al. found that resected pancreatic cancers in the tail were larger than those in the head, but they were also significantly less likely to have nodal metastasis (23). This raises the possibility that pancreatic tail cancers may constitute a more favorable malignancy than those located in the head. This disparity based on location has been seen in other sites that have more anatomically distinct compartments, like in colorectal cancers (24). Right and left-sided colon cancers, as well as proximal and distal rectal cancers, have different biological profiles; the conditions necessary to produce malignant potential proximally are intrinsically different conditions than distally (25). Recent studies in pancreatic cancers suggest that the location of the primary tumor is also associated with inherent differences in biological aggressiveness, in terms of recurrence and micrometastatic potential (26,27). At present, evidence is lacking to support or undermine the hypothesis that pancreatic tail cancers are truly less aggressive; it could just be that they have a different, albeit predictable, pattern of progression.
Regardless, pancreatic cancers are associated with high rates of clinically undetectable, micrometastatic disease (28-30). Perhaps pancreatic tail cancers are intrinsically more likely to progress through local growth and invasion due to a higher incidence of tumors with intact SMAD4 profiles. This could explain the significant survival benefit conveyed from the addition of adjuvant radiation. Further, this data suggests that prioritizing strategies that focus on improved local control with modern RT modalities may be particularly important for tumors in the tail of the pancreas.
Our study faced certain limitations from its inherent retrospective nature and relatively small sample size. We recognize that the median survival of 11.3 months in those patients who did not receive adjuvant RT is low and could reflect a cohort with increased co-morbidities that precluded adjuvant therapy integration. Indeed, the group of 14 patients who did not receive RT included 5 patients who did not receive any adjuvant therapy at all. We attempted to perform sub-group analysis to separate adjuvant chemotherapy as a possible confounder, but our study was not designed to do so. While seven patients received chemotherapy alone, no patients received adjuvant radiation alone. One patient received a significantly lower dose of radiation at our institution (9 Gy) because he was previously treated at a community hospital, where he was treated with a split course fractionation scheme. Otherwise, the small sample size precludes the retrospective elucidation of individual effects from chemotherapy and radiation.
Current studies suggest patients with pancreatic head tumors that are node positive or resected with positive margins benefit from adjuvant regimens that contain chemoradiation (31). This small study suggests that there may be improved survival for tail tumors with adjuvant RT, even for R0 resected tumors that are node negative. Moreover, the majority of the patients receiving adjuvant RT did so without any additional systemic chemotherapy, suggesting there may be increased benefit to intensifying local therapy integration in this subset of patients. Further study of pancreatic tail tumors in the adjuvant setting is warranted.
Conclusions
This study showed an improvement in survival with the addition of adjuvant RT for pancreatic tail tumors. The data suggests that tumors of the pancreatic tail compared with other locations may have a different genetic tumor profile favoring a higher propensity for local rather than distant disease progression. These findings need further validation to identify the most appropriate patient selection factors for adjuvant RT regimens for resected tail tumors.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This research was approved by the ethics committee and informed consent was obtained from all patients per our IRB# Pro 00003328 (0.02).
References
- Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009;6:699-708. [Crossref] [PubMed]
- Lau MK, Davila JA, Shaib YH. Incidence and survival of pancreatic head and body and tail cancers: a population-based study in the United States. Pancreas 2010;39:458-62. [Crossref] [PubMed]
- Li Y, Tang CG, Zhao Y, et al. Outcomes and prognostic factors of patients with stage IB and IIA pancreatic cancer according to the 8th edition American Joint Committee on Cancer criteria. World J Gastroenterol 2017;23:2757-62.
- Sener SF, Fremgen A, Menck HR, et al. Pancreatic cancer: a report of treatment and survival trends for 100,313 patients diagnosed from 1985-1995, using the National Cancer Database. J Am Coll Surg 1999;189:1-7. [Crossref] [PubMed]
- Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg 2015;220:530-6. [Crossref] [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [Crossref] [PubMed]
- Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer 1987;59:2006-10. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Abrams RA, Lillemoe KD, Piantadosi S. Continuing controversy over adjuvant therapy of pancreatic cancer. Lancet 2001;358:1565-6. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 2008;299:1019-26. [Crossref] [PubMed]
- Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol 2011;18:1319-26. [Crossref] [PubMed]
- Showalter TN, Zhan T, Anne PR, et al. The influence of prognostic factors and adjuvant chemoradiation on survival after pancreaticoduodenectomy for ampullary carcinoma. J Gastrointest Surg 2011;15:1411-6. [Crossref] [PubMed]
- Berger AC, Garcia M Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol 2008;26:5918-22. [Crossref] [PubMed]
- Berger AC, Winter K, Hoffman JP, et al. Five year results of US intergroup/RTOG 9704 with postoperative CA 19-9</=90 U/mL and comparison to the CONKO-001 trial. Int J Radiat Oncol Biol Phys 2012;84:e291-7. [Crossref] [PubMed]
- Bachet JB, Marechal R, Demetter P, et al. Contribution of CXCR4 and SMAD4 in predicting disease progression pattern and benefit from adjuvant chemotherapy in resected pancreatic adenocarcinoma. Ann Oncol 2012;23:2327-35. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [Crossref] [PubMed]
- Eschrich SA, Pramana J, Zhang H, et al. A gene expression model of intrinsic tumor radiosensitivity: prediction of response and prognosis after chemoradiation. Int J Radiat Oncol Biol Phys 2009;75:489-96. [Crossref] [PubMed]
- Strom T, Hoffe SE, Fulp W, et al. Radiosensitivity index predicts for survival with adjuvant radiation in resectable pancreatic cancer. Radiother Oncol 2015;117:159-64. [Crossref] [PubMed]
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1028-61. [Crossref] [PubMed]
- Evans DB, Hess KR, Pisters PW. ESPAC-1 trial of adjuvant therapy for resectable adenocarcinoma of the pancreas. Ann Surg 2002;236:694-author reply 694-6; author reply 694-6. [Crossref] [PubMed]
- Ruess DA, Makowiec F, Chikhladze S, et al. The prognostic influence of intrapancreatic tumor location on survival after resection of pancreatic ductal adenocarcinoma. BMC Surg 2015;15:123. [Crossref] [PubMed]
- Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 2017;28:1713-29. [Crossref] [PubMed]
- Slattery ML, Wolff E, Hoffman MD, et al. MicroRNAs and colon and rectal cancer: differential expression by tumor location and subtype. Genes Chromosomes Cancer 2011;50:196-206. [Crossref] [PubMed]
- Park J, Lee KT, Jang TH, et al. Risk factors associated with the postoperative recurrence of intraductal papillary mucinous neoplasms of the pancreas. Pancreas 2011;40:46-51. [Crossref] [PubMed]
- Khashab MA, Shin EJ, Amateau S, et al. Tumor size and location correlate with behavior of pancreatic serous cystic neoplasms. Am J Gastroenterol 2011;106:1521-6. [Crossref] [PubMed]
- Jang JY, Kang JS, Han Y, et al. Long-term outcomes and recurrence patterns of standard versus extended pancreatectomy for pancreatic head cancer: a multicenter prospective randomized controlled study. J Hepatobiliary Pancreat Sci 2017;24:426-33. [Crossref] [PubMed]
- Hurt CN, Falk S, Crosby T, et al. Long-term results and recurrence patterns from SCALOP: a phase II randomised trial of gemcitabine- or capecitabine-based chemoradiation for locally advanced pancreatic cancer. Br J Cancer 2017;116:1264-70. [Crossref] [PubMed]
- Kang CM, Hwang HK, Park J, et al. Maximum Standard Uptake Value as a Clinical Biomarker for Detecting Loss of SMAD4 Expression and Early Systemic Tumor Recurrence in Resected Left-Sided Pancreatic Cancer. Medicine (Baltimore) 2016;95:e3452. [Crossref] [PubMed]
- Merchant NB, Rymer J, Koehler EA, et al. Adjuvant chemoradiation therapy for pancreatic adenocarcinoma: who really benefits? J Am Coll Surg 2009;208:829-38; discussion 838-41. [Crossref] [PubMed]