Percutaneous irreversible electroporation with systemic treatment for locally advanced pancreatic adenocarcinoma
Introduction
Pancreatic adenocarcinoma has the highest mortality rate of all solid cancers and is the fourth leading cause of cancer death in Western countries (1,2); the outcome remains poor with an overall 5-year survival of less than 5% with no significant improvement over the last 50 years. Surgical resection offers the potential for cure, but less than 20% of patients are suitable and the median survival remains under 2 years and the 5-year survival ranging 11–21% (3,4). In the majority who present with metastatic (MPC) or inoperable locally advanced (LAPC) disease, the median survival ranges from 6–16 months. Considerable efforts have been made over the last decade to identify more effective systemic treatments (5-7). Unfortunately, most clinical trials have not shown any survival advantage for newer therapies including antiangiogenic approaches (8,9).
Local approaches for LAPC such as radiofrequency ablation appear promising, but being thermal-based and limited with significant risk of injury to adjacent blood vessels, gastrointestinal tract perforation and pancreatitis. Combining chemotherapy with RFA has been tried with results similar to CRT (10,11).
A more recent ablative technology, Irreversible Electroporation (IRE) is a non-thermal technique using ultra-short strong electrical fields to create permanent lethal nanopores in the cell membrane, disrupting cellular homeostasis and leading to apoptosis. The main advantage of IRE is in the conservation of blood vessel and bowel walls’ integrity. There are early reports of improved survival following percutaneous or intra-operative IRE-ablation in LAPC (12,13).
We assessed the safety and efficacy of percutaneous IRE integration in consecutive patients with unresectable locally advanced pancreatic adenocarcinoma (LAPC) undergoing chemotherapy.
Methods
Patients
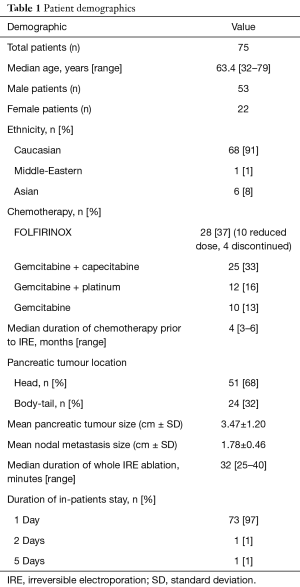
A retrospective analysis of patients with unresectable locally advanced pancreatic adenocarcinoma (LAPC) undergoing chemotherapy, treated with IRE-ablation, was performed. IRE-ablation was undertaken in a single centre with chemotherapy delivered at multiple referring hospitals. Exemption was granted by the internal review board as data review was retrospective (The Princess Grace Hospital, Reg: FV11042016). Data management was conducted within the requirements of the Data Protection Act. From the database of 75 consecutive patients with pancreatic carcinoma which remained stable after chemotherapy were treated with IRE between April 2011 and July 2016; LAPC tumours measured less than 5 cm and regional lymph nodes (number <3 with suspected involvement) measured less than 3 cm each. All cancers were ductal adenocarcinomas confirmed by biopsy or FNA. Patients had a minimum of 3 months of a standard first-line chemotherapy as dictated suitable by the referring oncologist (Table 1). All had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1, coagulation and platelet profile suitable for an interventional procedure; all had shown evidence of initial disease control after chemotherapy (stability/response by RECIST criteria).
Full table
All patients underwent contrast-enhanced computed-tomography (CT) of the chest and abdomen to assess the size, location and extension of the pancreatic carcinoma and to evaluate the degree of involvement of the adjacent vessels, common bile duct, duodenum/stomach and adjacent nodal metastases. CT/positron emission tomography (CT-PET) imaging was used if there was any doubt, to determine the extent of nodal disease. Contrast-enhanced magnetic resonance imaging (MRI) scans were carried out in select cases for more accurate staging.
IRE procedure
NanoKnife™ (AngioDynamics®, NY, USA) was used to carry out the IRE percutaneously using CT guidance, 2–7 days before or after the chemotherapy cycle, without interrupting standard-of-care chemotherapy as deemed safe by the referring oncologist. IRE was delivered by a generator supplying low-energy direct current at very high voltages (up to 3,000 V) (14,15). Two IRE 19-gauge monopolar needles spaced at 2 cm (with 2.5 cm exposure lengths) are positioned in parallel in the target tumour. An AccuSync (AccuSync Medical Research Corporation, Milford, CT) device was used to synchronize with the R wave of the electrocardiogram to deliver the planned 90 electrical pulses at a pulse-length of 70 µsec leading to an electrical current ranging 20–40 Amperes. The 2 electrodes were positioned in multiple overlapping axial planes serially to cover the whole tumoral volume (including a safety ablation-margin of 3–5 mm). Under GA and respiratory suspension following neuromuscular blockade, the needles were inserted percutaneously with no incision using a planned trajectory avoiding visceral and vessels walls. Prophylactic intravenous antibiotic (usually cefuroxime and/or metronidazole) was administered during the procedure. Subsequently 10 mL of Bupivacaine (50 mg) was instilled in the retro-crural space using a Chiba needle. For those with nodal metastases, the latter were ablated during the same session as the pancreatic carcinoma.
Follow-up
All patients were admitted overnight post-IRE. Complications were reported and scored retrospectively using NCI Common Terminology Criteria for Adverse Events version 4.0 (CTCAE). They were discharged the following morning. A follow-up contrast-enhanced CT scan of the abdomen was carried out before discharge in some cases to exclude sub-clinical adverse events.
Patients were followed up in the clinic within 2 weeks to assess toxicity and safety of proceeding with their planned chemotherapy, by the referring oncologist. Repeat CT scans of the chest and abdomen were performed two/three monthly after IRE. Post-IRE follow-up CT-PET scans were performed in some patients.
Statistics
Normally distributed variables are reported as percentage, mean and standard deviation (SD). Non-parametric variables are described as median and range. Kaplan-Meier curves were used to estimate the survival rate probability as median estimate [95% confidence interval (CI): lower – upper bound]. Survival was calculated from the time of IRE treatment and each patient was censored up to the time of last follow-up. Statistical analyses were performed using SPSS® software 22 (IBM corporation, Armonk, NY, USA).
Results
Seventy-five consecutive patients with disease control for unresectable locally advanced pancreatic carcinoma using IRE were analysed retrospectively. Patient demography and characteristics are summarised in Table 1. Prior to IRE, all LAPC were deemed unresectable at MDT review, except six patients who had previously failed an attempt at radical surgery. Everyone had chemotherapy before IRE [median duration: 4 (range, 3–6) months] and after IRE. Forty-three percent received FOLFIRINOX chemotherapy and the remainder received gemcitabine alone or gemcitabine-based combinations. Four patients had prior radical radiation therapy to the pancreas. At diagnosis and the time of IRE, major vascular invasion was noted with 52% involving the coeliac axis (CA) alone, 19% with superior mesenteric artery (SMA) alone, 12% with CA & SMA combined, 6% with SMA & superior mesenteric vein (SMV) combined, and 11% with CA/SMA + portal vein & SMV combined. The average duration of the IRE-ablation (measured from planning CT completion under GA to withdrawal of the IRE and Chiba needles) was 32 (range, 25–40) minutes.
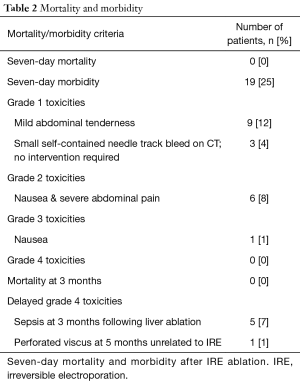
The 7-day mortality and morbidity after IRE-ablation was 0% and 25% respectively (Table 2). Twelve hours after the IRE-ablation, 3 patients (4%) had CT evidence of small self-contained bleed as a result of needle puncture requiring no intervention. Suspected IRE-ablation related adverse events and grades are shown in Table 2, as per CTCAEs. Five patients developed sepsis at 3 months following liver ablation and one patient had a reported perforated stomach at 5 months post-IRE which was unrelated to IRE.
Full table
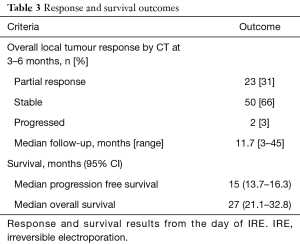
The median follow-up of the patients was 11.7 (range, 3–45) months. CT scans at 2–3 months after IRE-ablation showed a partial response of the treated pancreas mass (RECIST) in 31%; stable disease in 66% and local progression in 3%. Four of the patients with LAPC responded and down-staged to undergo Whipples operation, but one failed to proceed to resection, due to presence of peritoneal seedlings at laparotomy. However, the other 3 had complete R0 (pathological margin free of tumour) resections. Overall 38% of patients developed recurrent disease over 2 years with 25% in the liver, 10% in the peritoneum and 3% in the laparotomy cutaneous scar. The median change in the ratio of post IRE CA19-9 to Pre-IRE CA19-9 was 80% (range, 20–445%).
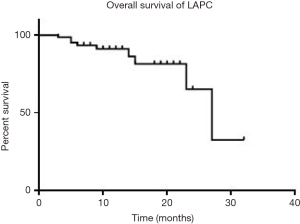
Survival outcomes from the day of IRE are summarised in Table 3. For patients with LAPC median OS was 27 months and the median progression-free survival (PFS) was 15 months (Figure 1).
Full table
Discussion
This is the largest series of IRE of unresectable pancreatic carcinoma carried out percutaneously to date. Whilst the main limitation is the retrospective nature, potentially leading to selection bias and variability of consistency of data, the overall survival (OS) of LAPC, as a non-variable end-point is encouraging. Selection bias was minimised by assessing a consecutive series. Patients who were progressing on treatment prior to referral were excluded; these are invariably poor candidates for consolidative or locally ablative treatments.
The results confirm the safety of IRE-ablation in previously studies (12,15-19). To date there has been no mortality directly attributed to the procedure using the percutaneous approach. Of the 43 patients undergoing 50 sessions of IRE ablation of the pancreatic carcinoma, Venkat and colleagues reported the complications of abdominal pain (23%), pancreatitis (14%), haematoma (16%), 1 case each (2%) of spontaneous pneumothorax, duodenal stenosis, portal vein thrombosis and sepsis (18). In the PANFIRE study of 25 LAPC cases, 11 patients developed gastrointestinal grade-I/II adverse events, 9 developed grade-III complications (2 pancreatitis, 3 biliary obstruction requiring stenting, 1 cholangitis requiring percutaneous drainage, 1 high grade SMA stenosis) and 2 grade-IV adverse events (1 pancreatitis and 1 bleeding from duodenal ulcer) (19). These complications might be explained by the larger tumour size in the cohort and possibly being more locally invasive. In our series, we reported 21% symptomatic cases of abdominal pain and nausea (1 patient required extended admission of 2 days) mostly of grade 1 and 2 toxicity and 3% asymptomatic (3 small haematoma requiring no intervention) cases within the first 7 days’ post-IRE ablation (Table 2). Delayed adverse events rate occurred in 9% (6 grade 4 and 3 grade 1) and we did not observe any symptomatic pancreatitis.
The alternative approach of intra-operative (open) IRE of LAPC has been reported before percutaneous IRE was developed (20-22). In the latest study with intra-operative IRE ablation of LAPC alone, 54 patients (36%) developed 100 complications (mostly of low grade toxicity) and of these the most common were gastrointestinal in origin in 70% of patients and 28% developed infection (23). There was 2% mortality within the 90-day follow-up with one patient bleeding from duodenal ulcer at day-55, one of liver failure at day-45 and another from pulmonary embolism on day-50 after IRE-ablation. There was no pancreatic-related complication, no leak or pancreatitis, as in our percutaneous cohort.
Outcome data from the Miami group on 43 patients treated with IRE percutaneously, following multiple treatments, showed a median OS of 14.5 months from the date of the IRE for those with LAPC (n=30) compared with 8.6 months for those with MPC (n=13); 95% had received pre-IRE chemotherapy and 44% received post-IRE chemotherapy (20). There was no reported PFS data. In the PANFIRE study, the median time to local progression after percutaneous IRE was 12 months (95% CI: 8–16 months). The median OS was 11 months from IRE (95% CI: 9–13 months) and 17 months from diagnosis (95% CI: 10–24 months). However, only 52% of those patients had received chemotherapy prior to IRE and tumour sizes were on the larger size for any ablative technique (the median size of 4 cm). The largest series of 200 intraoperative (open) IRE-ablated LAPC patients showed a median PFS of 12.4 months, distant PFS of 16.8 months and median OS of 24.9 months from time of diagnosis and a range of 18 to 23 months from day of IRE-ablation (23).
Similarly, our data from the day of percutaneous IRE of LAPC patients, the median PFS and OS were 15 and 27 months respectively (and 30 months from time of diagnosis). These are remarkably similar across different studies. Whilst none of these studies are randomised, multi-centred or controlled, there appears to be some consistency of data, and a possible survival benefit from IRE with acceptable morbidity irrespective of the method used (percutaneously or intra-operatively). Of note is the fact that our percutaneous non-invasive approach allowed for shorter procedural duration (32 vs. 195 minutes), reduced in-patient stay (1 vs. 6 days) and fewer IRE needles used (2 vs. 4), which would translate into lower costs, improved health economics and patient convenience.
The clinical utility of local radical chemo-radiotherapy (CRT) remains controversial. It may have a selective consolidating role after chemotherapy induction in those who have initial disease control (potentially allowing for a chemotherapy free-interval). A recent international phase-III study (LAP 07), showed no significant benefit in either the median PFS or OS comparing the addition of CRT versus chemotherapy alone in patients with inoperable LAPC (24). These outcomes are similar to the UK multicenter SCALOP study where the best median OS was 15.2 months (25). However, radical chemoradiotherapy is usually delivered over a seven-week period with at least 25 hospital-visits for treatment; re-admission due to toxicities and prolonged fatigue is common. Four of our IRE-treated LAPC patients had active disease by CT-PET following chemoradiotherapy and went on to have IRE-ablation successfully. Intriguingly, 4 IRE-treated LAPC patients were downstaged to resectability, when this had not been achieved prior to IRE with any prior treatment strategy. Similarly, 2 of 30 LAPC patients from the Miami study went on to have surgery after IRE ablation with R0 resection (18).
Conclusions
The data suggest that the integration of IRE-ablation within systemic chemotherapy is safe and potentially effective with improved survival for those patients with inoperable pancreatic carcinoma. These data provide a compelling argument to assess IRE plus chemotherapy against current standards of care in inoperable pancreatic cancer, within a prospective randomized phase III clinical trial.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Exemption was granted by the internal review board of The Princess Grace Hospital (Reg: FV11042016) as data review was retrospective.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer, 2013. Available online: http://globocan.iarc.fr
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [Crossref] [PubMed]
- Bockhorn M, Uzunoglu FG, Adham M, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2014;155:977-88. [Crossref] [PubMed]
- Diener MK, Fitzmaurice C, Schwarzer G, et al. Pylorus-preserving pancreaticoduodenectomy (pp Whipple) versus pancreaticoduodenectomy (classic Whipple) for surgical treatment of periampullary and pancreatic carcinoma. Cochrane Database Syst Rev 2014;(11):CD006053. Update in Cochrane Database Syst Rev 2016;2:CD006053. [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- da Rocha Lino A, Abrahão CM, Brandão RM, et al. Role of gemcitabine as second-line therapy after progression on FOLFIRINOX in advanced pancreatic cancer: a retrospective analysis. J Gastrointest Oncol 2015;6:511-5. [PubMed]
- Gunturu KS, Yao X, Cong X, et al. FOLFIRINOX for locally advanced and metastatic pancreatic cancer: single institution retrospective review of efficacy and toxicity. Med Oncol 2013;30:361. [Crossref] [PubMed]
- Nedaeinia R, Avan A, Manian M, et al. EGFR as a potential target for the treatment of pancreatic cancer: dilemma and controversies. Curr Drug Targets 2014;15:1293-301. [Crossref] [PubMed]
- Zagouri F, Sergentanis TN, Chrysikos D, et al. Molecularly targeted therapies in metastatic pancreatic cancer: a systematic review. Pancreas 2013;42:760-73. [Crossref] [PubMed]
- Cantore M, Girelli R, Mambrini A, et al. A triple approach strategy for patients with locally advanced pancreatic adenocarcinoma. J Clin Oncol 2011;29:e14607. [Crossref]
- Frigerio I, Girelli R, Giardino A, et al. Short term chemotherapy followed by radiofrequency ablation in stage III pancreatic cancer: results from a single center. J Hepatobiliary Pancreat Sci 2013;20:574-7. [Crossref] [PubMed]
- Narayanan G, Hosein PJ, Arora G, et al. Percutaneous irreversible electroporation for downstaging and control of unresectable pancreatic adenocarcinoma. J Vasc Interv Radiol 2012;23:1613-21. [Crossref] [PubMed]
- Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol 2013;20 Suppl 3:S443-9. [Crossref] [PubMed]
- Faroja M, Ahmed M, Appelbaum L, et al. Irreversible electroporation ablation: is all the damage nonthermal? Radiology 2013;266:462-70. [Crossref] [PubMed]
- Bagla S, Papadouris D. Percutaneous irreversible electroporation of surgically unresectable pancreatic cancer: a case report. J Vasc Interv Radiol 2012;23:142-5. [Crossref] [PubMed]
- Scheffer HJ, Vogel JA, van den Bos W, et al. Comment to: Månsson C, Nilsson A, Karlson B-M. Severe complications with irreversible electroporation of the pancreas in the presence of a metallic stent: a warning of a procedure that never should be performed. Acta Radiologica Short Reports 2014;3(11):1-3. Acta Radiol Open 2015;4:2058460115584111. [PubMed]
- Månsson C, Bergenfeldt M, Brahmstaedt R, et al. Safety and preliminary efficacy of ultrasound-guided percutaneous irreversible electroporation for treatment of localized pancreatic cancer. Anticancer Res 2014;34:289-93. [PubMed]
- Venkat S, Hosein PJ, Narayanan G. Percutaneous Approach to Irreversible Electroporation of the Pancreas: Miami Protocol. Tech Vasc Interv Radiol 2015;18:153-8. [Crossref] [PubMed]
- Scheffer HJ, Vroomen LG, de Jong MC, et al. Ablation of Locally Advanced Pancreatic Cancer with Percutaneous Irreversible Electroporation: Results of the Phase I/II PANFIRE Study. Radiology 2017;282:585-97. [Crossref] [PubMed]
- Bower M, Sherwood L, Li Y, et al. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J Surg Oncol 2011;104:22-8. [Crossref] [PubMed]
- Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg 2012;215:361-9. [Crossref] [PubMed]
- Paiella S, Butturini G, Frigerio I, et al. Safety and feasibility of Irreversible Electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Dig Surg 2015;32:90-7. [Crossref] [PubMed]
- Martin RC 2nd, Kwon D, Chalikonda S, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Ann Surg 2015;262:486-94; discussion 492-4. [Crossref] [PubMed]
- Hammel P, Huguet F, Van Laethem JL, et al. Comparison of chemoradiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: Final results of the international phase III LAP 07 study. J Clin Oncol 2013;13:abstr S89.
- Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013;14:317-26. [Crossref] [PubMed]