Non-surgical management of patients with intrahepatic cholangiocarcinoma in the United States, 2004–2015: an NCDB analysis
Introduction
Intrahepatic cholangiocarcinoma (ICC) is a rare neoplasm originating from the biliary epithelium of the liver that represents less than two percent of overall human cancers (1). It is associated with a poor prognosis owing to rapid tumor progression, biliary obstruction resulting in liver failure, and a low rate of surgical resection once diagnosed (2). Due to the relative rarity of ICC and its propensity to be diagnosed when unresectable, randomized control trials evaluating non-surgical therapies have not been performed. While surgical resection is the only therapy with a documented clinically meaningful survival benefit (3), prior retrospective database reviews estimate surgical intervention is provided in only 12% of cases; in fact, even patients less than 65 years of age with locally advanced disease only receive surgical resection 45% of the time (2). Patients who are not surgical candidates often receive palliative systemic chemotherapy with relatively poor response rates (4). Cisplatin plus gemcitabine produces a modest but statistically significant survival benefit of 8 months compared to 5 months with gemcitabine alone (5).
With technological advances in radiation oncology and ablation technologies, alternative methods are increasingly utilized for local tumor control. This rationale for liver-directed therapies is underscored by data that show survival benefit and decreased risk of liver failure in patients who receive local therapy with resection or definitive radiotherapy (6). To that end, multiple modalities have been heterogeneously employed for local tumor control. For patients with small (<3 cm) or intermediate (3–5 cm) sized inoperable tumors, percutaneous options like radiofrequency ablation (RFA) and microwave ablation (MWA) have been employed and demonstrate high rates of local tumor control and prolonged survival (7,8). While cholangiocarcinoma is generally regarded as a hypovascular tumor, transarterial chemoembolization and yttrium-90 (90Y) embolization have been utilized as bridging therapy to transplant or as definitive therapy in multiple small prospective studies (9). In addition to radioactive isotopes such as 90Y, radioactive brachytherapy implants have been employed for liver malignancies in single institution studies (10,11) with no formal studies on their efficacy in ICC (12). Finally, external beam radiation has been employed as a method of local tumor control, most commonly using traditional stereotactic body radiotherapy for small tumors (<5 cm) and complex dosing patterns for patients with larger tumors (>7 cm) (13,14). Unfortunately, many of these interventions and associated outcomes are documented only as institutional experiences.
The NCDB is a hospital-based, national registry of de-identified cancer patients and a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The present, retrospective study analyzed the largest cohort of ICC patients to date and characterized the demographic and clinical differences in patients managed non-surgically versus those managed surgically. It examined the same clinicopathologic data for patients managed non-surgically stratified by no local therapy, RFA, radioactive implant (RI) or external beam radiation. Finally, we reported overall survival between the non-surgical treatment options stratified by clinical stage for patients with ICC.
Methods
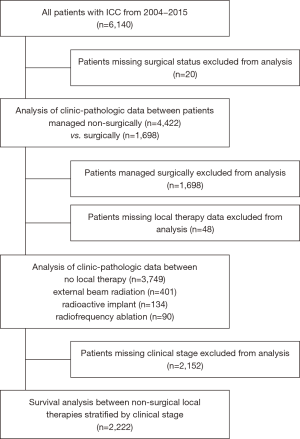
A retrospective review of the National Cancer Database (NCDB) was performed; the already de-identified database is considered Institutional Review Board (IRB) exempt. The participant user file analyzed in this study included all patients diagnosed with primary liver cancer from 2004 through 2015 with follow up to 2016. Patients with missing variables were excluded throughout the analysis. During the 11-year period, 6,140 patients diagnosed with histologically proven ICC in the NCDB were eligible for analysis. Patients were further stratified as to receiving surgery or no surgery; these groups were compared for various available demographic and clinicopathologic data. Surgery was defined as any combination of wedge or segmental resection, lobectomy, extended lobectomy, hepatectomy, or transplant. Clinical stage was defined based on the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control guidelines, which have been associated with better prognostic discrimination than prior staging criteria (15). Patients were further stratified into groups of no local therapy, external beam radiation (XRT), RI, RFA and compared for various available clinicopathologic data. External beam radiation was defined as X-ray, cobalt, linear accelerator, neutron beam, betatron, spray radiation and stereotactic radiosurgery including gamma knife and proton beam. RIs included brachytherapy, interstitial implants, molds, seeds, needles, and intracavitary applicators of radioactive materials or isotopes such as cesium, yttrium, strontium and gold. Thus, both brachytherapy and radioembolization procedures such as 90Y were coded together. RFA included both RFA and MWA. Other local tumor destruction methods such as photodynamic therapy, electrocautery, cryosurgery, alcohol ablation or trans-arterial chemotherapy embolization were either not available in the database or excluded. A detailed inclusion diagram is shown in Figure 1.
Statistical analysis was conducted using IBM SPSS Statistics (IBM Corp., Armonk, NY, USA) version 23. Variables were analyzed by exact Chi-square test for categorical variables or independent samples t-test for continuous variables. Survival curves were estimated using Kaplan-Meier methodology and differences between groups assigned using the log-Rank test for patients who received no local therapy, XRT, RI, or RFA stratified by clinical stage. Time-specific mortality rates for 1-, 3-, and 5-year mortality were calculated using life tables. Finally, pairwise comparisons were made between each treatment subgroup and evaluated by Kaplan-Meier analysis using the log-rank test. A P value <0.05 was considered statistically significant.
Results
Patient population and demographics
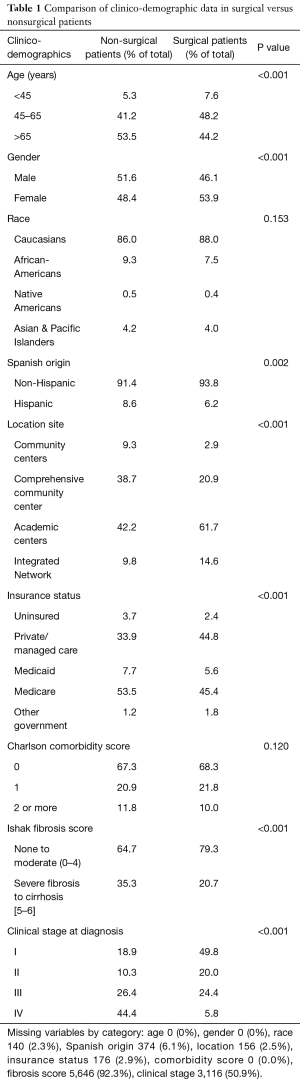
Of the 171,013 patients with primary liver cancer in the NCDB, 6,140 had ICC (3.6%). Of these patients, 4,374 (71%) did not undergo any surgery. Baseline demographic and clinical data of the patients who underwent surgery versus those who did not is presented in Table 1; a total of 6,120 patients were included in the final analysis. Age played a significant role in surgical status, with a higher proportion of non-surgical patients presenting over the age of 65 compared to surgical patients (53.5% vs. 44.2%, P<0.001). Patients managed non-surgically were slightly more likely to be male compared to surgical patients (51.6% vs. 41.6%, P<0.001). Patients managed non-surgically were much more likely to be at comprehensive community centers compared to patients who underwent surgery (38.7% vs. 20.9%) and less likely to be at academic centers (42.2% vs. 61.7%, P<0.001). Patients who did not undergo surgery were also more likely to have private insurance and less likely to have Medicare (P<0.001). Charlson-comorbidity score was not significantly different between surgical and non-surgical patients. A significantly greater proportion of non-surgically managed patients had Ishak fibrosis scores consistent with severe fibrosis or cirrhosis (35.3% vs. 20.7%, P<0.001). Patients managed non-surgically were significantly less likely to present with stage I disease compared to surgical patients (18.9% vs. 49.8%, P<0.001). Predictably, significantly fewer patients with stage IV disease were managed surgically compared to those that were not (5.8% vs. 44.4%, P<0.001).
Full table
Demographics of patients stratified by non-surgical treatment type
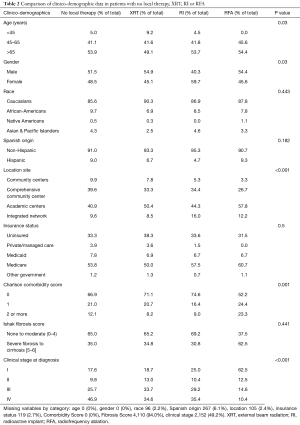
Comparison of clinicopathologic data of non-surgically managed patients who underwent no local therapy, XRT, RI, or RFA is presented in Table 2. There were 4,374 patients included in the analysis. Race, Hispanic origin, insurance status, and Ishak fibrosis score were not found to be statistically different between treatment types. There was a modest but statistically significant effect of age on treatment type, with a higher proportion of patients undergoing XRT and no patients undergoing RFA under age 45 (none 5.0%, XRT 9.2%, RI 4.5%, RFA 0%, P=0.03). A higher proportion of patients treated with RI were female compared to other treatments (none 48.5%, XRT 45.1%, RI 59.7%, RFA 45.6%, P=0.03). A higher proportion of patients treated with XRT, RI and RFA presented to academic centers (none 40.9%, XRT 50.4%, RI 44.3%, RFA 57.8%) compared to comprehensive community centers (none 39.6%, XRT 33.3%, RI 34.4%, RFA 26.7%, P<0.001). There was a higher proportion of patients treated with RFA with Charlson-comorbidity scores of 2 or more compared to other treatment types (none 12.1%, XRT 8.2%, RI 9.0%, RFA 23.3%, P=0.001).
Full table
Finally, clinical stage at diagnosis demonstrated the greatest differences in treatment type. Patients treated with no local therapy had a proportionally higher clinical stage at diagnosis. There was a higher proportion of patients who received no local therapy with stage IV disease (none 46.9%, XRT 34.6%, RI 35.4%, RFA 10.4%, P<0.001). Patients who underwent RFA were much more likely to present with stage I disease than the other treatment groups (none 17.6%, XRT 18.7%, RI 25.0%, RFA 62.5%, P<0.001).
Survival by non-surgical treatment type stratified by stage
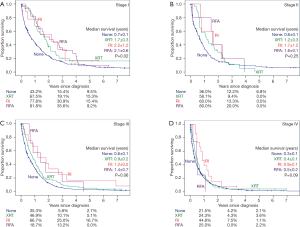
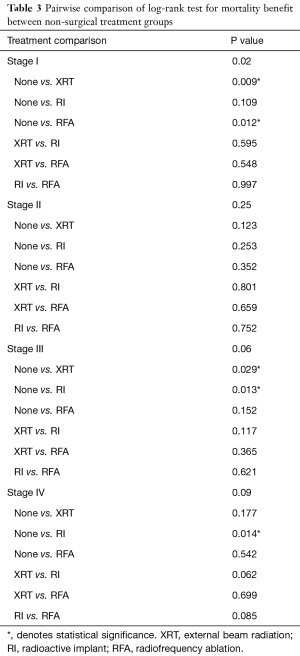
Given the significant differences in treatment type by clinical stage, patients were stratified by clinical stage to determine the impact of no local therapy, XRT, RI, and RFA on survival (Figure 2) with pairwise comparisons between treatment types (Table 3). There were 2,222 patients available for analysis. Throughout the analysis, the majority of patients received no local therapy. In stage I disease (Figure 2A), a statistically significant mortality benefit was demonstrated for patients receiving XRT, RI or RFA over no local therapy (P=0.002). Upon pairwise analysis, this difference resulted from the survival benefit of RFA over no therapy (median survival of 2.1 vs. 0.7 years, P=0.012) and XRT over no therapy (median survival 1.7 vs. 0.7 years, P=0.009). Patients with stage II disease (Figure 2B) demonstrated no mortality benefit with any Kaplan-Meier analysis or pairwise comparison. Patients with stage III (Figure 2C) disease demonstrated no overall mortality benefit between groups; however, subgroup analysis revealed a mortality benefit of XRT over no local therapy (median survival 0.9 vs. 0.6 years, P=0.029) and RI over no local therapy (median survival 1.2 vs. 0.6 years, P=0.013). Patients with stage IV disease (Figure 2D) demonstrated no overall differences in overall Kaplan-Meier analysis. Pairwise comparison revealed a significant mortality benefit for RI over no therapy (median survival 0.9 vs. 0.3 years, P=0.014).
Full table
Discussion
Despite surgical resection being the gold standard for cure, ICC is a relatively rare but aggressive primary liver neoplasm that is largely managed non-surgically. With advances in local tumor control techniques pioneered by both radiation oncologists and interventional radiologists, interdisciplinary physician teams have more tools at their disposal for patients in whom surgery is not indicated. However, no consensus guidelines exist for choosing among these various treatment modalities. Utilizing a large national cancer database to evaluate approximately 6,140 patients with ICC over an 11-year period, this study provides a unique snapshot into the non-surgical management of patients with cholangiocarcinoma in the United States. We demonstrate that patients managed non-surgically are typically older, treated at community centers and present with higher stage disease. We also show that patients managed non-surgically who either receive no local therapy, external beam radiation, RI, or RFA have unique demographic and pathologic characteristics, the most notable being clinical stage at diagnosis. Finally, we provide treatment-specific survival data stratified by clinical stage. To our knowledge, this is the largest study examining no local therapy, external beam radiation, RI, and RFA in patients with ICC.
We demonstrate that patients with ICC managed non-surgically are a unique population compared to their surgically-managed counterparts; they tend to be older with Medicare versus private insurance, receive treatment at community centers, and are proportionally more likely to have severe fibrosis or cirrhosis with higher stage disease at diagnosis. Interestingly, Charlson-comorbidity score index was not found to be a significant predictor of surgical status. When stratified by non-surgical treatment type, several patterns emerged. Patients were more likely to receive XRT, RI, or RFA at academic centers. Additionally, Charlson-comorbidity score showed a significant difference between groups, with a disproportionately high proportion of patients treated with RFA having scores of 2 or more. Like the clinicopathologic data stratified by surgical status, clinical stage at diagnosis demonstrated significant differences in clinical stage by treatment type. Specifically, very few patients with stage III or IV disease were treated with RFA compared to XRT or RI. Predictably, a significantly higher proportion of patients given no local therapy were diagnosed with stage IV disease.
We also explored overall survival between non-surgical treatment types and demonstrated unique survival benefits for XRT, RI and RFA at different clinical stages. RFA was associated with a significant survival benefit only in stage I disease. This finding is supported by multiple single-institution studies which demonstrate similar 1-, 3-, and 5-year survival rates for patients with stage I disease treated with radiofrequency or MWA (7,16) and suggests a limited role of RFA in late stage disease. Additionally, XRT demonstrated a survival benefit in stage I disease which correlates with prior data demonstrating improved overall survival of patients with ICC across clinical stage (13). Interestingly, there was no overall survival benefit for any treatment type demonstrated in stage II disease. This was perhaps a function of a relatively low number of RI and RFA in this group. Interestingly, XRT showed a pattern consistent with improved survival on Kaplan-Meier analysis in stage II disease but was not found to be statistically significant, dropping under the survival curve for no local therapy at approximately 3.5 years after diagnosis. Under the AJCC staging system, stage II ICC can include a single tumor with vascular invasion or multiple tumors with or without vascular invasion (15), which may account for the heterogeneity of this group when stratified by local tumor control treatments. When evaluating patients diagnosed with stage III disease, both XRT and RI were associated with survival benefit over no local therapy. This suggests that image-guided RIs, including brachytherapy and 90Y can be utilized to prolong survival in patients with multiple tumors that involve local hepatic structures by direct invasion consistent with stage III disease. Finally, patients with stage IV disease demonstrated a small but significant mortality benefit of RI over no local therapy of just over 6 months. This suggests that local tumor control with tumor-directed RI even in patients with periductal invasion can improve survival. It should be noted that 5-year survival between stages was not significantly affected by XRT, RFA or RI. A recent article suggests a useful paradigm for choosing a liver-directed therapy depending on tumor size, vascularity and location (12). Future studies utilizing these factors, in addition to the AJCC staging criteria, should be compared across treatment types for prognosis since they are likely to guide clinical decision-making.
This study has several limitations, many of which result from being a secondary data source. For one, coding in the database makes it difficult to separate various types of RIs (such as brachytherapy from 90Y), which makes clinical interpretation of this group challenging. While the coding for these specific treatments is vague and perhaps error-prone from an initial coding standpoint, other studies of large cancer databases have encountered similar issues. One retrospective review of the Surveillance, Epidemiology and End Results (SEER) database found that 43 patients with ICC were treated with brachytherapy alone from 1988–2003, representing 0.6% of the study population (17). Our population of patients that received any form of RI is approximately 3.3%, suggesting that this treatment group is composed of patients treated with both brachytherapy and radioactive isotopes like 90Y. Further studies on brachytherapy versus treatment with 90Y, particularly in patients with stage III and IV ICC are needed. Secondly, the challenge in using any large national cancer database is limited control over local practices regarding any technically challenging treatment. While the absence of local recurrence and disease-free survival is lacking in the NCDB, the noted observation that most patients with cholangiocarcinoma die of tumor-related liver failure or direct vascular compromise and not extrahepatic distant metastasis (13) makes overall survival a good indicator of disease progression. The database is also limited in determining the impact of systemic chemotherapy on survival in the adjuvant and palliative setting, in which many of the patients in this study would fall into. Despite the use of such a large database, the relative rarity of ICC meant that patients missing clinical stage data could not be utilized for Kaplan-Meier analysis, which decreased the power of the study. It is likely that a larger retrospective cohort could demonstrate survival benefit of other treatment modalities, since many of the log-rank results were close to significance. Nevertheless, this study was able to capture the largest number of patients with ICC stratified in this way to date.
In summary, this retrospective study utilizes a large national cancer database to demonstrate that patients diagnosed with ICC managed non-surgically are unique based on their demographics and stage at diagnosis. We provide treatment specific survival data for patients managed non-surgically stratified by clinical stage for no local therapy, XRT, RI, and RFA. Each of the treatment modalities demonstrated significant survival benefit over no local therapy; however, this varied dramatically depending on clinical stage. These findings are significant for clinicians and researchers for several reasons. It confirms the limited but effective role of microwave and RFA for small ICC lesions in comparison to XRT, which showed benefit in multiple clinical stages. It also demonstrates that XRT and RI are associated with improved survival over no local therapy in stage III disease, but only RI is associated with improved survival in patients with stage IV disease. Ideally, randomized control trials comparing liver-directed therapies that stratify patients into different clinical or pathologic stages should be conducted on ICC. This will remain a significant challenge and will require innovative multi-center cooperation to achieve.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The University of Florida was granted access to the Liver PUF dataset between 2004 to 2015. PUF’s are entirely de-identified data files accessible at select Commission on Cancer approved institutions that are in full compliance with the privacy requirements of the Health Insurance Portability and Accountability Act of 1996 as described in the Standards of Privacy of Individually Identifiable Health Information; Final Rule (45 CFR Parts 160 and 164) and have been previously subject to peer review and approval by the NCDB. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin 2006;56:106-30. [Crossref] [PubMed]
- Tan JCC, Coburn NG, Baxter NN, et al. Surgical Management of Intrahepatic Cholangiocarcinoma - A Population-Based Study. Ann Surg Oncol 2008;15:600-8. [Crossref] [PubMed]
- Kelley ST, Bloomston M, Serafini F, et al. Cholangiocarcinoma: advocate an aggressive operative approach with adjuvant chemotherapy. Am Surg 2004;70:743-8; discussion 748-9. [PubMed]
- Eckel F, Schmid RM. Chemotherapy and targeted therapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Chemotherapy 2014;60:13-23. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Yamashita S, Koay EJ, Passot G, et al. Local therapy reduces the risk of liver failure and improves survival in patients with intrahepatic cholangiocarcinoma: A comprehensive analysis of 362 consecutive patients. Cancer 2017;123:1354-62. [Crossref] [PubMed]
- Kim JH, Won HJ, Shin YM, et al. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol 2011;196:W205-9. [Crossref] [PubMed]
- Han K, Ko HK, Kim KW, et al. Radiofrequency ablation in the treatment of unresectable intrahepatic cholangiocarcinoma: systematic review and meta-analysis. J Vasc Interv Radiol 2015;26:943-8. [Crossref] [PubMed]
- Al-Adra DP, Gill RS, Axford SJ, et al. Treatment of unresectable intrahepatic cholangiocarcinoma with yttrium-90 radioembolization: a systematic review and pooled analysis. Eur J Surg Oncol 2015;41:120-7. [Crossref] [PubMed]
- Ricke J, Wust P, Wieners G, et al. Liver malignancies: CT-guided interstitial brachytherapy in patients with unfavorable lesions for thermal ablation. J Vasc Interv Radiol 2004;15:1279-86. [Crossref] [PubMed]
- Tselis N, Chatzikonstantinou G, Kolotas C, et al. Computed tomography-guided interstitial high dose rate brachytherapy for centrally located liver tumours: a single institution study. Eur Radiol 2013;23:2264-70. [Crossref] [PubMed]
- Koay EJ, Odisio BC, Javle M, et al. Management of unresectable intrahepatic cholangiocarcinoma: how do we decide among the various liver-directed treatments? Hepatobiliary Surg Nutr 2017;6:105-16. [Crossref] [PubMed]
- Tao R, Krishnan S, Bhosale PR, et al. Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis. J Clin Oncol 2016;34:219-26. [Crossref] [PubMed]
- Crane CH, Koay EJ. Solutions that enable ablative radiotherapy for large liver tumors: Fractionated dose painting, simultaneous integrated protection, motion management, and computed tomography image guidance. Cancer 2016;122:1974-86. [Crossref] [PubMed]
- Farges O, Fuks D, Le Treut YP, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer 2011;117:2170-7.
- Yu MA, Liang P, Yu XL, et al. Sonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinoma. Eur J Radiol 2011;80:548-52. [Crossref] [PubMed]
- Shinohara ET, Guo M, Mitra N, et al. Brachytherapy in the treatment of cholangiocarcinoma. Int J Radiat Oncol Biol Phys 2010;78:722-8. [Crossref] [PubMed]


