Histological and immunohistochemical study of hepatoblastoma: correlation with tumour behaviour and survival
Introduction
Hepatoblastoma (HB) is the most common primary hepatic tumour accounting for 50% of malignant hepatic tumours in children (1), with an annual incidence of 1.2–1.5 cases per million population (2,3). There are different histological subtypes with varying prognosis. Of the various molecular pathways of tumourigenesis, Wnt/beta-catenin pathway has gained importance recently. A simple way of identifying derangement in this pathway is by applying immunohistochemical (IHC) marker beta-catenin. There are not many studies comparing the expression of beta-catenin with histological subtypes and survival outcome in HB and results of the existing studies are contradictory (4-6).
Progenitor cells which express CK19 and/or EpCAM (7) play an important role in development of normal liver. HB arises from precursor small cells which at various stages of differentiation give rise to various subtypes thus recapitulating normal development (8,9). Hence, tumour differentiation depends on the stage at which malignant transformation occurs. In addition, CK19 expression has been found to correlate with aggressive behaviour in HB (6) as in hepatocellular carcinoma (7). Hepatic progenitor cells with a proliferative phenotype are associated with expression of EpCAM, which is expressed in 70–80% of HBs (8). Since EpCAM expression is independent of previous cisplatin based chemotherapy, it can be used as a tumour marker and a potential target for immunotherapy (9).
This study aimed to look in detail the morphological subtypes and compare the IHC expression of CK19, beta-catenin and EpCAM with histological subtypes, tumour behaviour, response to chemotherapy and survival. To date, there are no studies that have analysed the IHC expression in pre- and post-chemotherapy specimens of HB in Indian population.
Methods
This study was approved by the Institutional Research and Ethics Committee. As this study did not involve human subjects directly and used the stored formalin fixed paraffin embedded tissue blocks, a waiver of consent was obtained. A total of 55 cases of HB diagnosed in the Department of Pathology, from January 2000 to March 2015 were included. Of these, 22 patients had only biopsies, 11 had only resections and in 22 patients both biopsy and resection materials were available.
Slides and blocks were retrieved. All specimens were fixed in 10% formalin, embedded in paraffin and four-micron thick sections were cut and stained with haematoxylin and eosin. Representative tumour blocks were selected for immunohistochemistry.
Relevant clinical details and serum levels of alpha fetoprotein (AFP) at the time of diagnosis, post-chemotherapy, post-operative and at last follow up and beta-hCG (human chorionic gonadotropin) at initial diagnosis were noted.
Radiological findings included size and location of tumour, focality and presence of metastasis. Wherever available, the PRETEXT (pretreatment extent of disease) staging was noted.
Pathological assessment
For resection specimens, size, appearance (solid/cystic) and presence or absence of tumour necrosis were noted. Histological parameters analysed in both biopsy and resection specimens included histological subtype, mitotic activity/10 high power fields (hpf) with a cut off value of 5/10 hpf to designate tumours into low or high mitotic categories, presence of extramedullary haematopoiesis (EMH) and steatosis within the tumour.
Histologically, tumours were classified into six major subtypes: pure fetal epithelial, mixed embryonal and fetal epithelial, macro trabecular, small cell undifferentiated (SCUD) and mixed epithelial and mesenchymal (MEM) with and without teratoid features (10). A tumour was assigned a category depending on the predominant epithelial subtype (≥60%) exhibited. Tumours with 60:40 ratios of two or more components were classified as mixed subtype. However, for final diagnosis and statistical analysis, tumours were classified as predominantly fetal epithelial (includes pure fetal and predominantly fetal), mixed epithelial (includes predominantly embryonal and mixed fetal and embryonal), macro trabecular, SCUD and MEM with and without teratoid features.
In post-chemotherapy resections percentage of viable tumour was assessed and graded as <25%, 25%–<50% and ≥50%. Effects of chemotherapy and presence of microvascular invasion (MVI) were documented. Surgical resection margin was measured microscopically and categorised as ≤0.5, 0.6–1 and >1 cm.
Immunohistochemistry
IHC staining for CK19, EpCAM and beta catenin were carried out on selected blocks of 37 and 30 cases in the pre- and post-chemotherapy groups respectively, using Ventana Benchmark XT (CK19—clone b170, 1:150 dilution; beta-catenin—clone 17C2, 1:100 dilution; EpCAM—clone VU-1D9, pre-diluted RTU, Novocastra, Leica Biosystems, Melbourne, Australia). For CK19, >5% of tumour cells expressing moderate or strong membranous staining was taken as positive. It was assessed in fetal and embryonal subtypes separately and graded as 5–25%, 26–50% and >50%. Nuclear ± cytoplasmic expression of beta-catenin in >5% cells with moderate/strong staining was taken as positive. Membranous staining for EpCAM was considered as positive and Spizzo’s (11) scoring system was used.
Statistical analysis
Description of data was summarised using frequency along with percentages for categorical variables and mean along with standard deviation for continuous variables using SPSS software Version Stata IC/13. Chi-square/Fisher’s exact test was used to compare the association between categorical variables and P value of ≤0.05 was considered significant. Overall survival (OS) and event free survival (EFS) were calculated. Event was described as death/metastasis/recurrence. Kaplan-Meir curve was used to depict survival and log rank test was used to compare survival in different groups.
Results
Demographic and clinical details
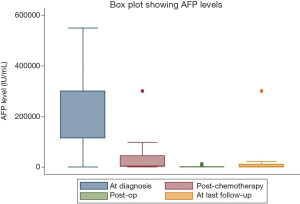
Mean (range) age of patients at presentation was 2.33±2.47 (0.05–13) years with a male: female ratio of 2.1:1 [37 and 18]. Most common clinical presentation was abdominal mass (38.2%) followed by fever (31%) and abdominal pain (14.6%). Two patients (3.6%) with HB were also found to have neuroblastoma. Other associations noted were horseshoe kidney in two patients, of which one also had medullary sponge kidney and gonadal enlargement with precocious puberty and gastroschisis in one each. Median (range) AFP level at diagnosis was 30,000 (4.32–549,400) IU/mL (n=46). One patient with normal serum AFP level (4.32 IU/mL) was diagnosed to have SCUD subtype. Decrease in serum AFP levels following chemotherapy and surgery was observed in 22/28 (78.6%) patients (Figure 1). Three patients had elevated serum beta-hCG at presentation and two died of disease.
Details about PRETEXT stage were available in 25 patients. Thirteen patients (52%) belonged to PRETEXT-II, 6 (24%) in PRETEXT-III and 3 patients each in PRETEXT-I (12%) and PRETEXT-IV (12%).
Of the 55 tumours, 30 (54.5%) were present in right lobe, 11 (20%) in left lobe and 14 (25.5%) involved both lobes. Of the 37 patients, 30 (81.1%) had a single tumour nodule, while 7/37 (18.9%) had multiple tumour nodules in the liver. Radiologically, average size of the tumour at initial presentation was 10.06±3.12 cm (n=43) and in post chemotherapy resection specimens (grossly) it was 6.70±2.89 cm. This difference in size between the two groups was statistically significant (P≤0.001) (n=25/33).
Gross details
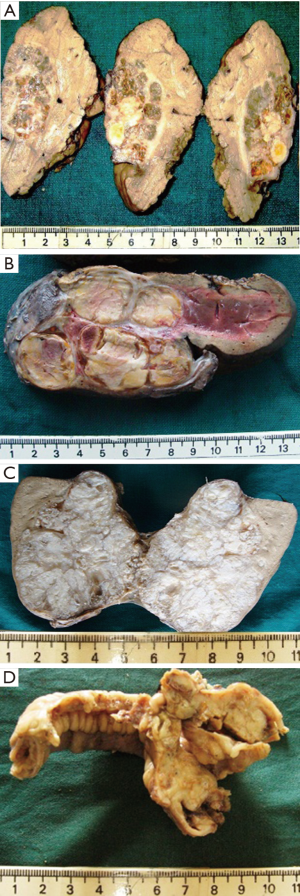
Of the 33 resected tumours, 28 (84.8%) had solid cut surface and 5 (15.2%) had both solid and cystic appearance. Necrosis was grossly evident in 8 (24.2%) cases. The tumours had a lobulated, firm, tan to grey white or variegated cut surface with haemorrhage, cystic change, hyalinisation and calcification or ossification (Figure 2A,B,C,D).
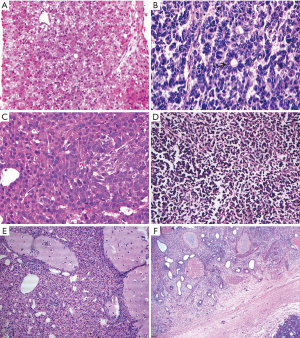
Microscopic findings (Figure 3A,B,C,D,E,F)
Most common epithelial subtype was fetal in 19/44 (43.2%) and mixed epithelial in 17/31 (54.8%) cases, in pre- and post-chemotherapy groups respectively. SCUD and macro trabecular subtypes were seen in one each.
EMH was noted in 21/44 (47.7%) and 24/33 (72.7%) tumours with erythroid most commonly and megakaryocytes in 3 and 6 tumours in pre- and post-chemotherapy groups respectively. Average number of mitosis/10 hpf was 2 and 1 in fetal and 6 and 11, in embryonal subtypes, in pre- and post-chemotherapy groups respectively. In pre-chemotherapy group, 85.3% and 36% of tumours in fetal and embryonal subtypes had a mitotic count of ≤5/10 hpf, respectively. One patient with fetal subtype had a mitotic count of 15/10 hpf and was categorised as ‘mitotically active fetal type’. But following chemotherapy, all (100%) fetal subtype tumours had a mitotic count of ≤5/10 hpf when compared to embryonal subtype (47%) (P≤0.001).
Steatosis was seen in 14/44 (31.8%) and 7/33(21.2%) tumours in pre- and post-chemotherapy groups respectively with all tumours in pre-chemotherapy group, belonging to predominantly fetal subtype (100%) (P=0.03). Osteoid was noted in 8/44 (18.2%) and 25/33 (75.8%) tumours in pre- and post-chemotherapy groups respectively. MVI was present in 14/33 (42%) resected tumours and these patients had lower EFS (46.2 months) when compared to those without (72.8 months) (P=0.08). Details of various subtypes of HB are shown in Table 1.

Full table
Following chemotherapy, 19/33 (55.6%) patients had ≥50% viable tumour. It was also found that 10/11 (90.9%) cases of MEM subtype and one case with SCUD subtype had ≥50% viable tumour (P=0.07). One tumour had extensive ossification with a few viable islands of normal looking hepatocytes (highlighted by EpCAM), indicating that these islands are actually tumour cells. When percentage of viable tumour was correlated with intensity of expression of EpCAM, 93.9% of cases with intense expression had ≥50% viable tumour (P=0.04).
Chemotherapy induced changes noted were hyalinisation, ossification, recent and old haemorrhage, necrosis, giant cell reaction, squamous differentiation and calcification. Margin could be measured for 30 tumours, of which 22 (73.4%) had a margin clearance of ≤0.5 cm, 4 (13.3%) 0.6–1 cm and 4 (13.3%) with >1 cm clearance. Only 18/22 patients with margin ≤0.5 cm had follow-up data available and 6 patients (33.3%) died of disease. Recurrence was found in 3/55 patients (one MEM and two mixed epithelial subtype) with a margin of ≤0.5 cm in the resected tumour. Distant metastasis was documented in 10/55 (18.2%) patients, most common site being the lung in 5/10 (50%) cases. Other sites of metastasis were omentum, ileum, lymph nodes, mesentery, diaphragm, brain and bones.
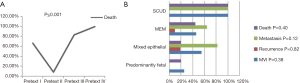
Survival data was available for 35/55 patients and 15 (42.9%) died due to disease. It was found that higher number of patients in PRETEXT III (83.3%) and PRETEXT IV (100%) succumbed to the disease compared to those in PRETEXT I and PRETEXT II (P≤0.001) (Figure 4A). Patients with predominantly fetal subtype of tumour had comparatively decreased chance of MVI, recurrence, metastasis and death; however, it was not statistically significant (Figure 4B).
IHC expression of CK19, beta-catenin and EpCAM
Staining pattern of various subtypes for CK19, beta-catenin and EpCAM are depicted in (Figure 5A,B,C).

CK19 expression was found in 21.9% and 17.4% in fetal and 54.2% and 72.2% in embryonal subtypes with a significant difference in both pre (P=0.01) and post (P<0.001) chemotherapy groups. In pre-chemotherapy group, CK19 expression was present in >50% of cells in 46.2% of tumours with embryonal subtype, but 85.7% of tumours with fetal subtype showed expression only in <25% of tumour cells (P≤0.04).
Nuclear expression of beta-catenin expression was present in 48.7% and 57.1% of tumours in pre- and post-chemotherapy groups respectively with strong expression in majority of tumours.
EpCAM was expressed in 37/37(100%) and 23/28 (82.1%) tumours in pre- and post-chemotherapy groups, respectively and majority of tumours in both groups, 34/37 (91.9%) and 19/23 (82.6%) respectively showed intense expression. When percentage of viable tumour was correlated with intensity of expression of EpCAM, 93.9% of cases with intense expression had ≥50% viable tumour (P=0.04) (Figure 6). One case with good response to chemotherapy with predominantly ossified areas and few viable clusters resembling normal hepatocytes on H&E examination were found to be immunopositive for EpCAM.
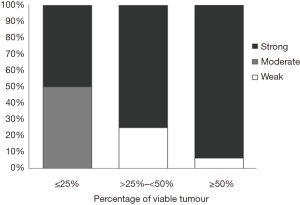
Comparison of IHC marker expression with tumour behaviour
Patients with embryonal subtype of tumour with CK19 expression had higher incidence of MVI, recurrence, metastasis and death. Increased mortality and adverse outcome was also seen in patients with EpCAM and beta-catenin (nuclear) expression. However, these differences were not statistically significant (Table 2).

Full table
Survival analysis
A total of 23 patients with follow up details available were taken for analysis. Overall median (range) survival was 70.7 (1.7 to 109.9) months and mean EFS was 59.5 months [95% confidence interval (CI): 35.7–83.3]. At 109 months (9 years) of follow-up, the estimated freedom from metastases was 56.5% (95% CI: 34.5–76.8%) (Figure 7A). The 5-year OS was 60%.
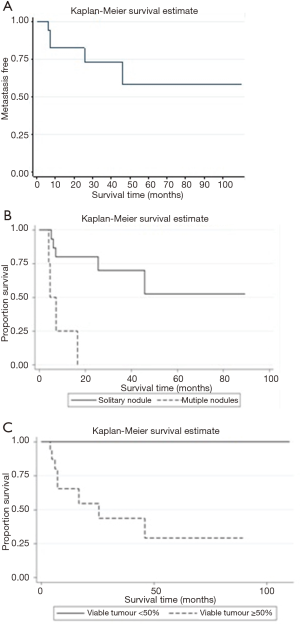
Kaplan-Meier survival estimates for EFS
There was a significant difference in EFS between patients with solitary tumour when compared to multiple nodules (P=0.001) and <50% viable tumour when compared to ≥50% viable tumour following chemotherapy (P=0.04) (Figure 7B,C). Other factors like age at diagnosis ≤2 years, male sex, AFP levels <10,000 IU/mL following chemotherapy, size ≤5 cm, PRETEXT I&II, mitosis ≤2/10 hpf and absent nuclear expression of beta-catenin also had higher EFS rates, though not statistically significant (Table 3).
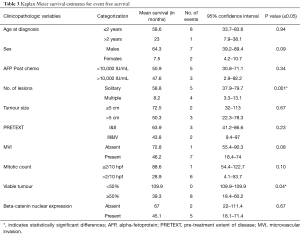
Full table
Discussion
HB is the most common primary tumour of liver affecting infants and children, with two-third of cases presenting in first 2 years and 90% occurring in the first 5 years of life (12,13). Mean age at presentation in this study was 2.3 years and 78% presented within 2 years of life as described previously (12). One patient in our study was diagnosed to have HB antenatally, which is a very rare presentation (14). Serum AFP, a very sensitive marker is elevated in 90% of HB patients (15) and 97.6% of patients in our study had an elevated AFP at presentation. Decrease in AFP level was noted following chemotherapy and surgery in 78.6% of patients (P≤0.001). Elevated beta-hCG is associated with poor prognosis (16) and in this study three patients had elevated levels of beta-hCG, of which two died of disease.
Better EFS was observed for patients in PRETEXT I & II, when compared to PRETEXT III and IV stages as described in other studies (17,18) and higher number of patients in PRETEXT III and IV succumbed to the disease when compared to PRETEXT I&II (P≤0.001).
Mean EFS of patients with multifocal tumour was only 8.2 months when compared to 58.8 months for those with a single tumour (P=0.001), which is similar to other studies (19). There was a significant decrease in tumour size following chemotherapy, indicating the chemosensitive nature of HB. Following chemotherapy, there is a substantial decrease in mitosis in fetal as compared to embryonal subtype (20) and in this study, all (100%) fetal and 47% embryonal tumours had mitosis of ≤5/10 hpf (P≤0.001). Osteoid was noted in 18.2% and 75.8% of tumours in pre- and post-chemotherapy groups respectively. Direct correlation between prognosis and proportion of osteoid has been documented previously (21) and two patients who had extensive ossification had no evidence of recurrence or metastasis. MVI was noted in 14 of 33 (42%) tumours and patients without MVI had EFS of 72.8 months when compared to 46.2 months in patients with MVI (22).
Comparing histological subtypes with adverse events, fetal subtype had a lower incidence of MVI, recurrence, metastasis and death which was similar to other studies (22,23). Patients with <50% of viable tumour following chemotherapy had higher EFS when compared to patients with >50% viable tumour (P=0.04). An interesting finding noted in this study was that majority of tumours with MEM subtype and the one with SCUD subtype had >50% viable tumour, indicating chemoresistance of these subtypes. SCUD pattern, even when present as a minor component, affects prognosis significantly (24) and in this study two cases with focal SCUD areas had MVI and metastasis, while the patient with pure SCUD subtype developed metastasis and succumbed to the disease. However, Gupta et al. (25) and Conran et al. (26) have found no significant association between histological subtype and survival.
When distance of the tumour from resection margin was compared with death, it was found that 33.3% of cases with margin ≤0.5 cm died of disease. In this study, it was also found that these patients had other features of prognostic importance like MVI (4 cases), SCUD (1case) subtype and lung metastasis (1 case), which can independently predict poorer outcome, irrespective of margin status (27,28).
CK19 expression was found in more number of embryonal (54.2% & 72.2%) subtype in pre- and post-chemotherapy groups respectively, when compared to fetal subtype (21.9% and 17.4%). This comparison was not done in previous studies. SCUD type also showed strong expression of CK19 and high frequency of expression of CK19 in embryonal and SCUD subtype confirms that it is a marker of embryonic stem cell and histogenesis of HB from embryonic cells (5). In this study, CK19 was found to be a marker of aggressiveness as described in other studies on HB (29) as well in hepatocellular carcinoma (7).
Nuclear expression of beta-catenin was present in 48.7% and 57.1% of tumours in pre- and post-chemotherapy groups respectively, which was similar to the study by Gupta et al. (25). A recent study (30) has documented weak beta-catenin expression in clear cell and hepatocellular carcinoma-like types of HB and strong expression in all pre-treated HBs. However, in this study, there was no significant difference between histological subtypes and beta-catenin expression. Results of comparison of beta-catenin expression with outcome are highly variable. In our study, patients with nuclear expression of beta-catenin were found to have decreased EFS (45.1 months), when compared to those without (66.7 months). Though Gupta et al. (25) found a relatively better prognosis with nuclear beta-catenin expression studies from Europe (31) and United States (20) have found no prognostic difference. These differences in expression of beta catenin could be explained based on the different genetic and environmental factors and also the multiple oncogenic pathways involving beta-catenin.
EpCAM expression was seen in 100% and 82.1% of tumours in pre- and post-chemotherapy groups respectively, which is similar to previous studies (4,32). Majority (>90%) of tumours with strong expression of EpCAM had ≥50% viable tumour following chemotherapy (P=0.04). To our knowledge, there is no data available in literature which correlates EpCAM expression and viability of tumour. Maturation of tumour cells is known to occur following chemotherapy and EpCAM expression would be useful in identifying these tumour clusters. In addition identifying EpCAM positive tumours may aid in targeted therapy with monoclonal antibodies improving the survival of those patients who are resistant to conventional chemotherapy (9).
Recurrence of tumour was found in 3 of 55 (5.5%) cases in our study and all three had persistent increase in AFP levels following chemotherapy/surgery. This can be used as a useful adjunct in monitoring patients for metastasis, recurrence and/or relapse (33,34). Distant metastasis was seen in 10 (18.2%) cases of which lung was the most common site, seen in 5 cases, as described previously (35). OS for patients who presented with lung metastasis was found to be lower (28.4 months) when compared to those without (75.9 months) (36,37).
Details about death was available for 35 of 55 cases, of which 15 (42.9%) died of disease. This was higher than North Indian (25) and German (38) studies, which documented only 21% and 23% deaths respectively. This may be due to more number of patients presenting at higher disease stage in our study. However, stage of disease at presentation was not mentioned in the previous studies.
Overall median survival of our patients was 70.7 months, and 5-year OS was 60% which is similar to other studies who have reported a 5-year OS of 63% (39) and 58.7% (40). Mean EFS in our study was 59.5 months and at 9 years of follow-up, estimated freedom from metastases was 56.5%, when compared to 77% metastasis free survival at 2 years of follow-up in a study by Wang et al. (41).
Although stage of tumour at diagnosis remains the key factor in determining prognosis, (26,42,43) various clinical and histological factors have also been implicated in prognostication (6,25,41,44-46). In this study, multifocality and percentage of viable tumour ≥50% were significant factors when EFS was compared. Other factors like age at diagnosis ≤2 years, male sex, AFP level <10,000 IU/mL following chemotherapy, size ≤5 cm, PRETEXT I&II, mitosis ≤2/10 hpf and absent nuclear expression of beta-catenin were also associated with higher EFS.
In conclusion, this study has looked at the histopathological subtypes of HB and compared the IHC expression of CK19, Beta-catenin and EpCAM with survival in 55 cases, diagnosed over a period of 16 years. A larger multicentre collaborative study is required for proper validation of results and also to identify potential biomarkers which can be useful in targeted therapy for HB.
Acknowledgements
We thank Mrs. Mahasampath Gowri. S, Department of Biostatistics, for her assistance in statistical analysis. This study was funded by the Fluid Research Fund, Christian Medical College, Vellore, India.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: This study was approved by the Institutional Review Board, Christian Medical College, Vellore, India (IRB Min No. 8985). As this study did not involve human subjects directly and used the stored formalin fixed paraffin embedded tissue blocks, a waiver of consent was obtained.
References
- Stocker JT. Hepatoblastoma. Semin Diagn Pathol 1994;11:136-43. [PubMed]
- von Schweinitz D. Hepatoblastoma: recent developments in research and treatment. Semin Pediatr Surg 2012;21:21-30. [Crossref] [PubMed]
- Park WS, Oh RR, Park JY, et al. Nuclear localization of beta-catenin is an important prognostic factor in hepatoblastoma. J Pathol 2001;193:483-90. [Crossref] [PubMed]
- Ward SC, Thung SN, Lim KH, et al. Hepatic progenitor cells in liver cancers from Asian children. Liver Int 2010;30:102-11. [Crossref] [PubMed]
- Abenoza P, Manivel JC, Wick MR, et al. Hepatoblastoma: an immunohistochemical and ultrastructural study. Hum Pathol 1987;18:1025-35. [Crossref] [PubMed]
- Yun WJ, Shin E, Lee K, et al. Clinicopathologic implication of hepatic progenitor cell marker expression in hepatoblastoma. Pathol Res Pract 2013;209:568-73. [Crossref] [PubMed]
- Matthai SM, Ramakrishna B. Cancer stem cells in hepatocellular carcinoma - an immunohistochemical study with histopathological association. Indian J Med Res 2015;142:391-8. [Crossref] [PubMed]
- de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, et al. Expression of EpCAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol 1999;188:201-6. [Crossref] [PubMed]
- Armeanu-Ebinger S, Hoh A, Wenz J, et al. Targetting EpCAM (CD326) for immunotherapy in hepatoblastoma. Oncoimmunology 2013;2:e22620. [Crossref] [PubMed]
- Ishak KG, Goodman ZD, Stocker JT. Atlas of Tumour Pathology: Tumors of the Liver and Intrahepatic Bile Ducts. Hepatoblastoma. Washington, DC: Amer Registry of Pathology, 2001.
- Spizzo G, Fong D, Wurm M, et al. EpCAM expression in primary tumour tissues and metastases: an immunohistochemical analysis. J Clin Pathol 2011;64:415-20. [Crossref] [PubMed]
- Schmidt D, Harms D, Lang W. Primary malignant hepatic tumours in childhood. Virchows Arch A Pathol Anat Histopathol 1985;407:387-405. [Crossref] [PubMed]
- Herzog CE, Andrassy RJ, Eftekhari F. Childhood Cancers: Hepatoblatoma. Oncologist 2000;5:445-53. [Crossref] [PubMed]
- Shih JC, Tsao PN, Huang SF, et al. Antenatal diagnosis of congenital hepatoblastoma in utero. Ultrasound Obstet Gynecol 2000;16:94-7. [Crossref] [PubMed]
- Schnater JM, Kohler E, Lamers WH, et al. Where do we stand with hepatoblastoma? A review. Cancer 2003;98:668-78. [Crossref] [PubMed]
- Nakagawara A, Ikeda K, Tsuneyoshi M, et al. Hepatoblastoma producing both alpha-fetoprotein and human chorionic gonadotropin. Clinicopathologic analysis of four cases and a review of the literature. Cancer 1985;56:1636-42. [Crossref] [PubMed]
- Meyers RL, Rowland JR, Krailo M, et al. Predictive power of pretreatment prognostic factors in children with hepatoblastoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2009;53:1016-22. [Crossref] [PubMed]
- Ismail H, Broniszczak D, Kalicinski P, et al. Changing treatment and outcome of children with hepatoblastoma: analysis of a single center experience over the last 20 years. J Pediatr Surg 2012;47:1331-9. [Crossref] [PubMed]
- Saettini F, Conter V, Provenzi M, et al. Is multifocality a prognostic factor in childhood hepatoblastoma? Pediatr Blood Cancer 2014;61:1593-7. [Crossref] [PubMed]
- Ranganathan S, Tan X, Monga SP. Beta-Catenin and met deregulation in childhood Hepatoblastomas. Pediatr Dev Pathol 2005;8:435-47. [Crossref] [PubMed]
- Heifetz SA, French M, Correa M, et al. Hepatoblastoma: the Indiana experience with preoperative chemotherapy for inoperable tumours; clinicopathological considerations. Pediatr Pathol Lab Med 1997;17:857-74. [Crossref] [PubMed]
- Kasai M, Watanabe I. Histologic classification of liver-cell carcinoma in infancy and childhood and its clinical evaluation. A study of 70 cases collected in Japan. Cancer 1970;25:551-63. [Crossref] [PubMed]
- Malogolowkin MH, Katzenstein HM, Meyers RL, et al. Complete surgical resection is curative for children with hepatoblastoma with pure fetal histology: a report from the Children's Oncology Group. J Clin Oncol 2011;29:3301-6. [Crossref] [PubMed]
- Haas JE, Feusner JH, Finegold MJ. Small cell undifferentiated histology in hepatoblastoma may be unfavorable. Cancer 2001;92:3130-4. [Crossref] [PubMed]
- Gupta K, Rane S, Das A, et al. Relationship of β-catenin and postchemotherapy histopathologic changes with overall survival in patients with hepatoblastoma. J Pediatr Hematol Oncol 2012;34:e320-8. [Crossref] [PubMed]
- Conran RM, Hitchcock CL, Waclawiw MA, et al. Hepatoblastoma: the prognostic significance of histologic type. Pediatr Pathol 1992;12:167-83. [Crossref] [PubMed]
- Dicken BJ, Bigam DL, Lees GM. Association between surgical margins and long-term outcome in advanced hepatoblastoma. J Pediatr Surg 2004;39:721-5. [Crossref] [PubMed]
- Schnater JM, Aronson DC, Plaschkes J, et al. Surgical view of the treatment of patients with hepatoblastoma. Cancer 2002;94:1111-20. [Crossref] [PubMed]
- Singh V, Manalang M, Singh M, et al. A Brief Report of Immunohistochemical Markers To Identify Aggressive Hepatoblastoma. Appl Immunohistochem Mol Morphol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Huang WJ, Tsai JH, Jeng YM. Complementary roles of β-catenin and glutamine synthetase immunostaining in diagnosis of chemotherapy-treated and untreated hepatoblastoma. J Formos Med Assoc 2017;116:549-53. [Crossref] [PubMed]
- Purcell R, Childs M, Maibach R, et al. Potential biomarkers for hepatoblastoma: results from the SIOPEL-3 study. Eur J Cancer 2012;48:1853-9. [Crossref] [PubMed]
- Ruck P, Wichert G, Handgretinger R, et al. EpCAM in malignant liver tumours. J Pathol 2000;191:102-3. [Crossref] [PubMed]
- Kubota M, Yagi M, Kanada S, et al. Effect of postoperative chemotherapy on the serum alpha-fetoprotein level in hepatoblastoma. J Pediatr Surg 2004;39:1775-8. [Crossref] [PubMed]
- Rojas Y, Guillerman RP, Zhang W, et al. Relapse surveillance in AFP-positive hepatoblastoma: re-evaluating the role of imaging. Pediatr Radiol 2014;44:1275-80. [Crossref] [PubMed]
- Emre S, Umman V, Rodriguez-Davalos M. Current concepts in pediatric liver tumours. Pediatr Transplant 2012;16:549-63. [Crossref] [PubMed]
- Wanaguru D, Shun A, Price N, et al. Outcomes of pulmonary metastases in hepatoblastoma – is the prognosis always poor? J Pediatr Surg 2013;48:2474-8. [Crossref] [PubMed]
- Perilongo G, Brown J, Shafford E, et al. Hepatoblastoma presenting with lung metastases. Cancer 2000;89:1845-53. [Crossref] [PubMed]
- Fuchs J, Rydzynski J, Von Schweinitz D, et al. Pretreatment prognostic factors and treatment results in children with hepatoblastoma: a report from the German Cooperative Pediatric Liver Tumour Study HB 94. Cancer 2002;95:172-82. [Crossref] [PubMed]
- Allan BJ, Parikh PP, Diaz S, et al. Predictors of survival and incidence of hepatoblastoma in the paediatric population. HPB (Oxford) 2013;15:741-6. [Crossref] [PubMed]
- Liu G, Liu B, Li K, et al. Outcome of hepatoblastoma: experience with 63 patients received chemotherapy with the regimen C5V. Zhonghua Er Ke Za Zhi 2015;53:119-23. [PubMed]
- Wang LL, Filippi RZ, Zurakowski D, et al. Effects of neoadjuvant chemotherapy on hepatoblastoma: a morphologic and immunohistochemical study. Am J Surg Pathol 2010;34:287-99. [Crossref] [PubMed]
- von Schweinitz D, Wischmeyer P, Leuschner I, et al. Clinico-pathological criteria with prognostic relevance in hepatoblastma. Eur J Cancer 1994;30A:1052-8. [Crossref] [PubMed]
- von Schweinitz D, Hecker H, Schmidt-von-Arndt G, et al. Prognostic factors and staging systems in childhood hepatoblastoma. Int J Cancer 1997;74:593-9. [Crossref] [PubMed]
- Maibach R, Roebuck D, Brugieres L, et al. Prognostic stratification for children with hepatoblastoma: the SIOPEL experience. Eur J Cancer 2012;48:1543-9. [Crossref] [PubMed]
- Qiao GL, Li L, Cheng W, et al. Predictors of survival after resection of children with hepatoblastoma: A single Asian center experience. Eur J Surg Oncol 2014;40:1533-9. [Crossref] [PubMed]
- Meyers RL, Maibach R, Hiyama E, et al. Risk-stratified staging in paediatric hepatoblastoma: a unified analysis from the Children’s Hepatic tumors International Collaboration. Lancet Oncol 2017;18:122-31. [Crossref] [PubMed]


