Gastrointestinal stromal tumours: advances in surgical and pharmacological management options
Background
Gastrointestinal stromal tumours (GISTs), first described by Mazur and Clark (1) are a group of mesenchymal neoplasms which arise from precursors of connective-tissue cells of the gastrointestinal tract (2). The incidence of GISTs is thought to be between 10 and 20/106 people per year (3,4). It has been estimated that around 70% of GISTs occur in the stomach, around 25% develop in the small intestine and less than 10% are present in other parts of the gastrointestinal tract (2), although other reports do vary slightly (5). Occasionally, GISTs can occur in the omentum, mesentery, or retro-peritoneum (6). Men and women are equally affected (7) and GISTs are diagnosed principally between the ages of 40 to 80 (8). However, GISTs can have a familial component and hence occur in younger patients (9).
Until recently, GISTs have been particularly problematic for decades, mainly due to the lack of diagnostic criteria related to a poor understanding of their origin and differentiation. This has consequently led to an inconsistent nomenclature over time, resulting in them being classified as leiomyosarcomas. Recent progress concerning the pathophysiology and management of GISTs indicates a morphological and immunophenotypical overstimulation of the pacemaker interstitial cells of Cajal (ICC). Over 90% of GIST and ICC express the tyrosine kinase receptor KIT (CD117), which is unanimously phosphorylated due to a gain-of-function mutation in the c-KIT proto-oncogene (7,10-12). A minority (<5%) of classic GISTs which lack c-KIT mutations express mutations in platelet-derived growth factor receptor alpha (PDGFRα), another tyrosine kinase receptor (13-15).
This has led to the remarkable development of the revolutionary tyrosine kinase-inhibiting agent imatinib mesylate (Gleevec) which was first utilised for GISTs in 2000 (16). In more recent times, sunitinib, also inhibiting VEGFR in addition to KIT and PDGFRα, has shown to be valuable in patients intolerant to imatinib (17). However, since GISTs represent a broad clinical spectrum from benign to highly malignant, treatment and prognosis also vary accordingly (18). Regarding operable GISTs, especially localised primary tumours, surgical resection remains the primary treatment modality and was traditionally the only effective intervention. Since around 40% to 90% of surgical patients develop subsequent postoperative recurrence or metastasis, the need for adjuvant and neo-adjuvant therapy has become fundamental to improve survival rates in surgically managed patients with GISTs.
This review article aims at reviewing the management of primary GISTs, investigating recurrences, exploring the role of surgery in metastatic disease and looking into combined surgery and molecular therapy for GISTs.
Primary GISTs
Management
As mentioned above, GISTs can occur anywhere in the GI tract, from the oesophagus to the rectum as well as the omentum, mesentery and retroperitoneum. The presence of KIT expression has also supported their existence in unusual sites like the gallbladder (19) and urinary bladder (20). Fewer than half of GIST patients present with localised primary tumours (21). Complete surgical resection is the first-line intervention in patients with primary GIST. The majority of GISTs arise from the muscularis propria of the stomach or intestinal wall and exhibit an exophytic growth pattern rather than infiltrating the organ, resulting in protrusion from the tissue of origin and explaining the fact that negative surgical margins of resection are relatively easy to obtain (7). The microscopic margin of resection is not correlated to survival, and is not deemed as important as whether tumour cells are transferred directly into the peritoneal cavity, although the relatively low number of subjects in this study by DeMatteo et al. forbids a final conclusion (8). For that reason, wedge resections or segmental resections of the underlying organ are commonly used to treat primary GISTs and Shiu et al. (22) even reported that wide resection had no known benefit. Complications can arise due to the fragile nature of these tumours, particularly in large ones or in cases of intratumoral haemorrhage or necrosis. Consequently, detailed and careful surgical technique to avoid intraoperative tumour rupture, which is a poor prognostic indicator, is imperative (23). Ng et al. (24) concluded that tumour rupture reduced the median survival from 46 to 17 months, a figure comparable to the median survival after incomplete resection (21 months). In their study, Yao et al. (25) reported a median survival for patients who underwent a complete resection to be 46 months compared to 10 months for those who underwent an incomplete resection. It was also stated that total gastrectomy or en bloc resection of adjacent organs could be needed in more extensive gastric lesions. In contrast to intestinal adenocarcinoma, GISTs seldom metastasise to lymph nodes, thereby eliminating the need for lymphadenectomy (6). This concurs with findings from Yao et al. who reported a low incidence of 7.7% of lymph node involvement (25). However, Roberts and Eisenberg advocate the need of a peritonectomy where appropriate due to the frequent occurrence of peritoneal seeding (26). It should be noted that current literature on the surgical management of primary GISTs distort actual results of resection and survival rates due to the fact that investigators have grouped together patients with primary and recurrent GIST, due to the relatively rare occurrence of the disease (6).
Prognosis
Bucher et al. (18) analysed the prognosis after primary GIST resection and concluded that low grade GISTs had an excellent prognosis after resection alone. However, high-grade GISTs were found to be associated with a high risk of recurrence after primary resection. This finding is consistent with a study by Yao et al. (25) who also identified unfavourable prognostic factors as incomplete resection, high-grade histologic features and tumour size of 5 cm or more (P<0.5). In addition, tumour grade was found to be the most important predictor in patients with complete resection (median survival, 55 vs. 19 months in 35 patients; P<0.05). Interestingly, Nilsson et al. (27) estimated a 10% reduced risk of dying in patients with gastric GIST compared with patients having small intestinal and colon GIST. Although tumour size, location, mitotic index (MI) and age can act as independent predicting factors for malignant potential in GISTs, a study on 1,004 subjects by Emory et al. (28) revealed that an accurate clinical prediction of the course of the disease still necessitated better methods. A summary of the outcomes after resection in primary GISTs is shown in Table 1 below.

Full table
Recurrence
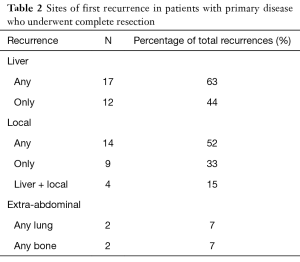
Complete surgical resection with pathologically established negative margins can still be followed by recurrence. In fact, the majority of tumours recur (29). It has been reported that patients possessing a mutation of exon 11 displayed higher rates of recurrence. Van der Zwan and DeMatteo (30) stated that there was no proven benefit of earlier detection of recurrent GISTs to improve survival. At the Roswell Park Cancer Institute, recurrence rates of 76% for gastric tumours and 64% for small and large bowel tumours were reported. Recurrence was noted in 80% of patients during follow-up after complete resection in a study by Ng et al. (24). In a retrospective study at the Memorial Sloan-Kettering Cancer Centre on 60 patients, local recurrence was determined in 76% of patients (31). Interestingly, a much lower figure of 44% was reported by Conlon et al. (32) who also noted a mean time to recurrence of 9 months (median 7, range <1–37). Recurrence usually involves the liver and peritoneum surface. In their study of 80 patients with primary presentation who underwent complete resection, DeMatteo et al. (8) concluded that the liver was involved in 63% of patients with recurrence and was the sole area of disease in 44% cases of recurrence. A more detailed description of the published data on sites of first recurrence is shown in Table 2 below. Nilsson et al. (27) reported a satisfactory process of stratification of GISTs into very low-risk, intermediate risk, high-risk groups and their own addition of an overtly malignant group to predict the outcome regarding recurrent tumours and survival.
Full table
Metastatic GIST
All GISTs should be regarded as having a malignant potential, with the possible exclusion of very small tumours measuring <1 cm (30). In the past, patients with advanced GIST (unresectable or metastatic disease) have had a very poor prognosis associated with a median survival of around 1.5 years after surgical resection (8). Most patients eventually develop tumour recurrence, with a median time to recurrence after surgery reported to be between 18–24 months (30,33). Much focus has been placed on studies to outline criteria to discern benign GIST from malignant GIST (34). Tumour size and mitotic rate have been consistently identified as the most reliable predictors so far (27). However, certain cases of very small and mitotically inactive GISTs which subsequently followed a malignant path have been reported (30,35). In a similar fashion to primary tumours, DeMatteo et al. (8) noted that out of 94 patients presenting with metastasis, 65% had liver disease and 53% had isolated liver recurrence. These liver metastases are traditionally multifocal although around 26% of patients have resectable disease (30). An analysis of 131 subjects with metastatic GIST to liver, hepatic resection was achieved in 34 of them. The 1- and 3-year survival rates were 90% and 58%, respectively. An important predictor of survival was the time span between treatment of the primary disease and the development of liver metastasis (33). The likelihood of ensuing extra-abdominal metastases (e.g., lung or bone) later in the progression of the disease has been mentioned in literature (30).
It has been shown that surgery on its own or coupled with conventional chemotherapy or radiation therapy has been majorly ineffective in treating most patients with malignant GIST, with a terribly low response rate of 5% in conventional chemotherapy (6,8). This has led to imatinib being the first-line treatment of metastatic GIST. However, surgery can still play an invaluable role in certain cases. Symptomatic patients presenting with primary GIST with synchronous, low-volume metastatic disease localised to a single site (e.g., liver) are eligible to be considered for surgical resection first (21,30). Resection of multiple intra-abdominal organs and surgery for debulking is not advocated and provides no significant benefit and is further contraindicated in patients with poor performance status and comorbidity (21,36). In metastatic GIST, disease control is achieved by imatinib in approximately 80% of patients, and interestingly, the 2-year survival in advanced GIST is now around 75% to 80% (16,37). An amount of 400 mg daily is widely regarded as the standard starting dose, although the optimal dose of imatinib has not been determined to date. Tumour density is considered as a better marker of treatment response than tumour size alone (38). However, most patients ultimately develop resistance to imatinib (39). Less than 15% of patients display primary (immediate) resistance to imatinib, while others subsequently acquire resistance after achieving stable disease, classically after more than one year of therapy.
Surgery and pharmacological therapy
The recent progress in understanding the pathophysiology behind GISTs has driven forward interest in them. As a result, the development of highly specific targeted therapy with imatinib mesylate has seen the light (27). Imatinib selectively inhibits particular tyrosine kinases, including KIT, PDGFRα and others. It has revolutionised the treatment of metastatic GISTs, with a partial response or stable disease in up to 80% of patients (30). According to NICE guidelines, imatinib was granted European marketing authorisation in May 2002 to treat adult patients with KIT-positive unresectable and/or metastatic GIST (40). The median time to progression on imatinib is 2 years (37). Albeit the recent suggestions that a minority of the much rarer KIT-negative tumours may also respond to imatinib, treatment for this group of tumours is not warranted in the UK (37). Surgery is not warranted for patients with metastatic GIST and multifocal resistance (more than 1 tumour increasing in size) (40).
The success of imatinib therapy in the treatment of metastatic GIST has aroused interest in its perioperative efficacy. This would include both preoperative reduction in size of tumours as well as adjuvant therapy following complete resection of primary tumours. The American College of Surgeons Oncology Group (ACSOG) has recently undertaken a Phase II intergroup trial evaluating the value of adjuvant imatinib at a dose of 400 mg/day for 12 months following complete surgical resection in patients having high-risk primary GIST. Inclusion criteria for high-risk GIST included tumour size ≥10 cm, intraperitoneal tumour rupture or haemorrhage, or multifocal (<5) tumours. The single-arm, open-label study of 107 subjects concluded that a prolonged 2-year overall survival of 97% was achieved, compared to the previous figure of around 50% before the availability of imatinib (41).
The potential cure provided by surgical resection offers the justification for the use of imatinib pre-operatively to shrink previously inoperable GISTs. This neoadjuvant approach would potentially optimise the timing of surgery, avoid emergencies and reduce the risk of complications in patients with large GISTs at risk of haemorrhage or rupture (21). Bümming et al. (42) reported the case of a 56-year-old man presenting with unresectable primary epithelioid GIST originating from the ileum with liver and mesenteric metastases. After starting imatinib treatment, tumour regression to half its original dimensions and the occurrence of liver metastases into cystic masses were noted. Complete surgical resection of the tumour was undertaken, sparing the rectum, bladder and prostate. Stable partial response was shown by subsequent PET and CT scans.
Conclusions
The outlook on GISTs has undergone an immense metamorphosis over the years. The recent increase in knowledge on the pathobiology of these tumours and their consistent nomenclature has opened up new doors for their management, previously entirely dependent on surgical resection. For patients with resectable GISTs, surgery remains the primary treatment modality. However, the vast majority of these patients eventually develop recurrence after complete resection. Increased survival in GIST patients directly correlates with the outcome of surgery. The advent of imatinib as targeted cancer therapy has completely transformed the management of GISTs, dramatically improving patient outcomes. Imatinib has shown a remarkable capability to considerably reduce tumour size and manage metastatic GIST in patients with advanced disease and hence, could help reduce the risks of complications from surgical tumour resection, leading to an increase in survival. Very recent data by the ACSOG has shed long-awaited light on adjuvant imatinib therapy and has laid the foundations for an interesting future while neoadjuvant imatinib therapy has been shown to significantly prolong survival in patients with initially unresectable GISTs. However, much input still needs to be invested in research on the appropriate dose and duration of imatinib therapy and better understanding of second-line tyrosine kinase inhibitors is required due to imatinib resistance. It is now clear that in order to improve results in GIST treatment, a multimodality approach centred around surgery should be adopted.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7:507-19. [Crossref] [PubMed]
- Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: recent advances in understanding of their biology. Hum Pathol 1999;30:1213-20. [Crossref] [PubMed]
- Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol 2004;22:3813-25. [Crossref] [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch 2001;438:1-12. [Crossref] [PubMed]
- Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 2005;100:162-8. [Crossref] [PubMed]
- Dematteo RP, Heinrich MC, El-Rifai WM, et al. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol 2002;33:466-77. [Crossref] [PubMed]
- Kosmadakis N, Visvardis EE, Kartsaklis P, et al. The role of surgery in the management of gastrointestinal stromal tumors (GISTs) in the era of imatinib mesylate effectiveness. Surg Oncol 2005;14:75-84. [Crossref] [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [Crossref] [PubMed]
- Nishida T, Hirota S, Taniguchi M, et al. Familial gastrointestinal stromal tumours with germline mutation of the KIT gene. Nat Genet 1998;19:323-4. [Crossref] [PubMed]
- Lux ML, Rubin BP, Biase TL, et al. KIT extracellular and kinase domain mutations in gastrointestinal stromal tumors. Am J Pathol 2000;156:791-5. [Crossref] [PubMed]
- Rubin BP, Singer S, Tsao C, et al. KIT activation is a ubiquitous feature of gastrointestinal stromal tumors. Cancer Res 2001;61:8118-21. [PubMed]
- Corless CL, McGreevey L, Haley A, et al. KIT mutations are common in incidental gastrointestinal stromal tumors one centimeter or less in size. Am J Pathol 2002;160:1567-72. [Crossref] [PubMed]
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708-10. [Crossref] [PubMed]
- Kindblom LG, Remotti HE, Aldenborg F, et al. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998;152:1259-69. [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [Crossref] [PubMed]
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [Crossref] [PubMed]
- Demetri GD, Desai J, Fletcher JA, et al. SU11248, a multi-targeted tyrosine kinase inhibitor, can overcome imatinib (IM) resistance caused by diverse genomic mechanisms in patients with metastatic gastrointestinal stromal tumor (GIST). Proc Am Soc Clin Oncol 2004;22:3001. [Crossref]
- Bucher P, Egger JF, Gervaz P, et al. An audit of surgical management of gastrointestinal stromal tumours (GIST). Eur J Surg Oncol 2006;32:310-4. [Crossref] [PubMed]
- Ortiz-Hidalgo C, de Leon Bojorge B, Albores-Saavedra J. Stromal tumor of the gallbladder with phenotype of interstitial cells of Cajal: a previously unrecognized neoplasm. Am J Surg Pathol 2000;24:1420-3. [Crossref] [PubMed]
- Lasota J, Carlson JA, Miettinen M. Spindle cell tumor of urinary bladder serosa with phenotypic and genotypic features of gastrointestinal stromal tumor. Arch Pathol Lab Med 2000;124:894-7. [PubMed]
- Eisenberg BL, Judson I. Surgery and imatinib in the management of GIST: emerging approaches to adjuvant and neoadjuvant therapy. Ann Surg Oncol 2004;11:465-75. [Crossref] [PubMed]
- Shiu MH, Farr GH, Papachristou DN, et al. Myosarcomas of the stomach: natural history, prognostic factors and management. Cancer 1982;49:177-87. [Crossref] [PubMed]
- Kwon SJ. Korean Gastric Cancer Study Group. Surgery and prognostic factors for gastric stromal tumor. World J Surg 2001;25:290-5. [Crossref] [PubMed]
- Ng EH, Pollock RE, Munsell MF, et al. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 1992;215:68-77. [Crossref] [PubMed]
- Yao KA, Talamonti MS, Langella RL, et al. Primary gastrointestinal sarcomas: analysis of prognostic factors and results of surgical management. Surgery 2000;128:604-12. [Crossref] [PubMed]
- Roberts PJ, Eisenberg B. Clinical presentation of gastrointestinal stromal tumors and treatment of operable disease. Eur J Cancer 2002;38 Suppl 5:S37-8. [Crossref] [PubMed]
- Nilsson B, Bümming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 2005;103:821-9. [Crossref] [PubMed]
- Emory TS, Sobin LH, Lukes L, et al. Prognosis of gastrointestinal smooth-muscle (stromal) tumors: dependence on anatomic site. Am J Surg Pathol 1999;23:82-7. [Crossref] [PubMed]
- Pidhorecky I, Cheney RT, Kraybill WG, et al. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol 2000;7:705-12. [Crossref] [PubMed]
- van der Zwan SM, DeMatteo RP. Gastrointestinal stromal tumor: 5 years later. Cancer 2005;104:1781-8. [Crossref] [PubMed]
- Mudan SS, Conlon KC, Woodruff JM, et al. Salvage surgery for patients with recurrent gastrointestinal sarcoma: prognostic factors to guide patient selection. Cancer 2000;88:66-74. [Crossref] [PubMed]
- Conlon KC, Casper ES, Brennan MF. Primary gastrointestinal sarcomas: analysis of prognostic variables. Ann Surg Oncol 1995;2:26-31. [Crossref] [PubMed]
- DeMatteo RP, Shah A, Fong Y, et al. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg 2001;234:540-7; discussion 547-8. [Crossref] [PubMed]
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65. [Crossref] [PubMed]
- Miettinen M, El-Rifai W, H L, Sobin L, et al. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol 2002;33:478-83. [Crossref] [PubMed]
- Blanke CD, Eisenberg BL, Heinrich MC. Gastrointestinal stromal tumors. Curr Treat Options Oncol 2001;2:485-91. [Crossref] [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34. [Crossref] [PubMed]
- Choi H, Charnsangavej C, de Castro Faria S, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol 2004;183:1619-28. [Crossref] [PubMed]
- DeMatteo RP, Maki RG, Singer S, et al. Results of tyrosine kinase inhibitor therapy followed by surgical resection for metastatic gastrointestinal stromal tumor. Ann Surg 2007;245:347-52. [Crossref] [PubMed]
- National Institute for Health and Care Excellence Guidelines. Imatinib for the adjuvant treatment of gastrointestinal stromal tumours. Available online: https://www.nice.org.uk/guidance/ta326/documents/gastrointestinal-stromal-tumours-imatinib-adjuvant-rev-ta196-id696-final-appraisal-determination-document2
- DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinib mesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg 2013;258:422-9. [Crossref] [PubMed]
- Bümming P, Andersson J, Meis-Kindblom JM, et al. Neoadjuvant, adjuvant and palliative treatment of gastrointestinal stromal tumours (GIST) with imatinib: a centre-based study of 17 patients. Br J Cancer 2003;89:460-4. [Crossref] [PubMed]


