FOLFIRINOX treatment leading to pathologic complete response of a locally advanced pancreatic cancer
Introduction
Pancreatic cancer (PC) is a lethal disease and commonly asymptomatic. Therefore, it is most often diagnosed at an advanced stage (1). Symptoms arise if the tumor is located close to the common bile duct (CBD) causing obstructive jaundice. Up to date, PC is the fourth leading cause of cancer related deaths (2). Surgical resection is the single curative option (1,3). However, surgery is possible in less than 20% of the patients. A locally unresectable tumor is present in 30% of the cases, whereas the majority presents with distant metastases (1). Unresectable PC can be divided into locally advanced PC (LAPC) and borderline resectable PC (BRPC). LAPC is defined as a surgically unresectable tumor encasing the adjacent arteries [celiac axis, superior mesenteric artery (SMA)] or occluding the adjacent veins (portal vein, superior mesenteric vein) (4). BRPC is a tumor bordering less than 50% of the circumference of the SMA, celiac axis, and short segment abutment, encasement of the common hepatic artery or segmental venous occlusion.
Common treatment options comprise chemotherapy and chemoradiation therapy, which should be initiated rapidly for downstaging and treatment of occult micrometastases (5).
Standard chemotherapeutical regimens include FOLFIRINOX [folinic acid (leucovorin), fluorouracil, irinotecan and oxaliplatin], gemcitabine, nab paclitaxel, capecitabine, cisplatin, docetaxel (6-9). FOLFIRINOX treatment can improve the survival rate and radiologic response in patients with unresectable PC (10,11). Therefore, significant tumor response can turn palliative into neoadjuvant chemotherapy.
We report the case of a patient with pathologic complete response (pCR) following neoadjuvant chemotherapy with FOLFIRINOX for LAPC.
Case presentation
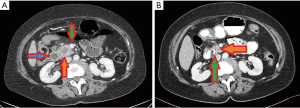
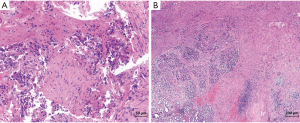
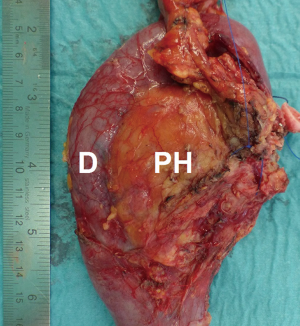
A 67-year old Caucasian female patient presented to our hospital complaining of painless jaundice as the only symptom. Medical history was significant for type 2 diabetes mellitus. Ultrasound revealed an anechoic lesion in the pancreatic head. Laboratory studies were remarkable for carbohydrate antigen (CA) 19-9 284.7 U/mL (<27 U/mL), gamma glutamyl transferase 112 U/L (10–71 U/L), alkaline phosphatase 301 U/L (40–129 U/L) and bilirubin 23.3 mg/dL (<1.1 mg/dL). Computed tomography showed a tumor in the pancreatic head infiltrating the portal vein and the gastroduodenal artery but no signs of abdominal or thoracic metastases (Figure 1). Endoscopic retrograde cholangiography (ERC) with stenting of the CBD was performed. Cytopathological findings revealed an adenocarcinoma (Figure 2). The patient received one cycle of FOLFOX (folinic acid 400 mg/m2, fluorouracil 2,400 mg/m2 and oxaliplatin 85 mg/m2) due to slightly elevated bilirubin and five cycles of FOLFIRINOX (folinic acid 400 mg/m2, fluorouracil 2,400 mg/m2, irinotecan 180 mg/m2 and oxaliplatin 85 mg/m2). Preoperative re-staging showed a significant tumor response without any signs of vessel infiltration. CA 19-9 level was within a normal range after chemotherapy. Hence, a pylorus-preserving pancreatectomy and splenectomy was performed (Figure 3). Intraoperative ultrasound of the liver ruled out liver metastases. Total pancreatectomy was required due to extremely soft pancreatic tissue, which allowed creation of a high risk pancreato-jejunal anastomosis only. Histopathological examination showed pCR of the PC. No viable tumor cells could be identified in the specimens. Tumor stage was ypT0, pN0 (0/35), M0, L0, V0, Pn0. The patient was discharged on postoperative day 20 in a good general condition. Subsequently, adjuvant FOLFIRINOX treatment was continued for two cycles. Restaging via CT scan 2 months after surgery showed no signs of tumor recurrence.
Discussion
Early diagnosis of PC remains challenging due to a typically asymptomatic course.
Locally unresectable PC (stage III) is present in 30%, whereas most patients suffer from metastasized PC at time of diagnosis. Less than 20% of the patients qualify for primary surgical resection (1,12). Median survival in the palliative setting reaches only 5–10 months, whereas neo- or adjuvant therapy can increase the survival up to 2 years after complete resection (1,13).
Approximately 30% of the patients with unresectable PC reach a status of resectability after neoadjuvant chemotherapy (8,9). Neoadjuvant treated patients have similar survival rates after R0 resection of PC in comparison to patients with primary resectable PC (9,14). Their median survival is 20.5–34.2 months after curative resection in contrast to 14 months in neoadjuvant treated patients without resectability (7,9). However, patients with margin positive resected PC have similar survival outcomes with those who were treated initially palliative (9). Conroy et al. showed a significant increase of median survival from 6.8 to 11 months in patients with metastatic PC after FOLFIRINOX treatment, which suggested better survival rates in patients with unresectable PC (10). Follow up studies confirmed FOLFIRINOX as a powerful first-line regimen in patients with initially unresectable cancer which can lead to resectability. FOLFIRINOX treatment can lead to R0 resection in up to 96% of patients with BRPC (7,8,15,16).
pCR following FOLFIRINOX treatment is extremely rare. It has been reported in 4.5–5.9% (8,15-18).
We report the rare case of pCR of a pancreatic head cancer after FOLFIRINOX treatment. Obstructive jaundice led to the initial diagnosis of a LAPC. Primary resection was not possible due to infiltration of the portal vein and the gastroduodenal artery. One cycle of FOLFOX treatment and five cycles of FOLFIRINOX treatment led to significant tumor downsizing enabling an uneventful pylorus preserving pancreatectomy. Adjuvant chemotherapy with the FOLFIRINOX regimen was continued for two cycles. A CT scan 2 months after surgical resection showed no evidence of tumor recurrence.
In our case FOLFIRINOX treatment was initiated in a palliative pattern and turned neoadjuvant due to significant tumor response.
Nevertheless, local tumor recurrence has been described in 30% after early treatment (1). Paniccia et al. observed occurrences of hepatic metastases 4 months after complete resection of a neoadjuvant treated pancreatic head cancer with pCR (15).
While pCR is an excellent outcome after neoadjuvant treated PC, adjuvant therapy and frequent follow up investigations are required since tumor recurrence remains a significant menace.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010;362:1605-17. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg 2004;91:586-94. [Crossref] [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [Crossref] [PubMed]
- Bond-Smith G, Banga N, Hammond TM, et al. Pancreatic adenocarcinoma. BMJ 2012;344:e2476. [Crossref] [PubMed]
- Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg 2008;206:833-46; discussion 46-8. [Crossref] [PubMed]
- Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85. [Crossref] [PubMed]
- Boone BA, Steve J, Krasinskas AM, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol 2013;108:236-41. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Pietrasz D, Marthey L, Wagner M, et al. Pathologic Major Response After FOLFIRINOX is Prognostic for Patients Secondary Resected for Borderline or Locally Advanced Pancreatic Adenocarcinoma: An AGEO-FRENCH, Prospective, Multicentric Cohort. Ann Surg Oncol 2015;22 Suppl 3:S1196-205. [Crossref] [PubMed]
- Mahipal A, Frakes J, Hoffe S, et al. Management of borderline resectable pancreatic cancer. World J Gastrointest Oncol 2015;7:241-9. [Crossref] [PubMed]
- Hartlapp I, Muller J, Kenn W, et al. Complete pathological remission of locally advanced, unresectable pancreatic cancer (LAPC) after intensified neoadjuvant chemotherapy. Onkologie 2013;36:123-5. [Crossref] [PubMed]
- Strobel O, Berens V, Hinz U, et al. Resection after neoadjuvant therapy for locally advanced, "unresectable" pancreatic cancer. Surgery 2012;152:S33-42. [Crossref] [PubMed]
- Paniccia A, Edil BH, Schulick RD, et al. Neoadjuvant FOLFIRINOX application in borderline resectable pancreatic adenocarcinoma: a retrospective cohort study. Medicine (Baltimore) 2014;93:e198. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist 2013;18:543-8. [Crossref] [PubMed]
- Moorcraft SY, Khan K, Peckitt C, et al. FOLFIRINOX for locally advanced or metastatic pancreatic ductal adenocarcinoma: the Royal Marsden experience. Clin Colorectal Cancer 2014;13:232-8. [Crossref] [PubMed]


