Second-line therapy in advanced upper gastrointestinal cancers: current status and new prospects
Introduction
Gastrointestinal malignancies are the most common cause of cancer worldwide (1). Upper gastrointestinal cancers (UGC) arise from the esophagus, stomach, pancreas and hepatobiliary system. Often, patients with UGC present with advanced incurable disease or relapse after initial surgery. For these patients, prognosis is dismal, and the goal of therapy is to palliate symptoms, improve quality of life (QoL) and prolong survival. Patient- and tumor-related factors, such as performance status (PS), comorbidities, organ function, tumor-related symptoms, tumor burden, histologic-molecular subtypes and doctor-patient preference, influence the therapeutic choice (2-4). Fewer than half of patients with UGC receive any additional treatment after progressing on frontline therapy (5-7). Hereafter, we provide an overview on second-line therapies for these patients and examine emerging strategies.
Second-line therapy: achievements and limitations
Esophagogastric cancer (EGC)
Chemotherapy
Fluoropyrimidines (FP), platinum compounds, taxanes, topoisomerase inhibitors and anthracyclines form the platform for treatment of patients with advanced OGC. Platinum and FP-based doublets (either alone or in combination with trastuzumab in HER2-overexpressing adenocarcinoma) or triplets are commonly used as first-line therapies (8,9). Three phase III trials support the use of chemotherapy as second-line treatment. The AIO trial compared irinotecan with best supportive care (BSC). Unfortunately, the study closed prematurely after randomization of 40 patients due to poor accrual (10). Overall survival (OS) was improved in the irinotecan arm (4.0 vs. 2.4 months; HR 0.48, P=0.012). In a larger Korean trial, patients (n=202) were assigned to receive either single-agent docetaxel or irinotecan plus BSC vs. BSC alone (11). OS was significantly improved in the chemotherapy arm (5.3 vs. 3.8 months; HR 0.65, P=0.009), and no difference has emerged between agents. With a similar design, in the COUGAR-2 study, patients (n=168) received docetaxel plus BSC vs. BSC alone (12). OS was superior in the docetaxel arm (5.2 vs. 3.6 months; HR 0.67, P=0.01). In a meta-analysis of these 3 trials, the risk of death was reduced in those treated with chemotherapy compared with BSC (HR 0.63, P<0.0001), and the benefit was observed regardless the chemotherapeutic agent (13). In a further phase III trial (n=223), paclitaxel was compared with irinotecan, and no difference in OS (9.5 vs. 8.4 months; HR 1.13, P=0.38) emerged between agents (14). Two additional phase III trials have been conducted in Japanese patients. In the first study, patients (n=130) refractory to S-1-based chemotherapy received irinotecan plus cisplatin or irinotecan alone (15). Progression-free survival (PFS), which served as the primary endpoint, was marginally improved in the doublet arm (3.8 vs. 2.8 months; HR 0.68, P=0.0398). However, this improvement did not translate into OS benefit. In the second trial, platinum-naïve patients (n=163) progressing on single-agent S-1 for metastatic disease or relapsing within 6 months after completion of S-1 adjuvant therapy were randomized to receive irinotecan plus cisplatin or irinotecan alone (16), and no difference in OS (13.9 vs. 12.7 months; HR 0.834, P=0.288) was detected. In a recent phase III trial (n=741), nab-paclitaxel was not inferior in terms of OS compared with standard paclitaxel (HR 0.97, non-inferiority one-sided P=0.0085). The response rate (RR) was in favor of nab-paclitaxel, and QoL was similar between the two arms (17). In a phase II trial with cabazitaxel for previously treated patients, the reported DCR was 20% in second-line and 30% in all lines in patients without prior taxane use. The median PFS was 2.01 months for patients not previously treated with taxanes (18).
Targeted agents
Vascular endothelial growth factor (VEGF)
In a phase III trial of first-line therapy, no survival benefit was observed with bevacizumab in addition to chemotherapy (19). Signals of the efficacy of VEGF blockade emerged with ramucirumab, a recombinant IgG1 monoclonal antibody class that binds to VEGF-2. Two phase III trials support the use of ramucirumab as second-line therapy. In the first study, patients (n=355) progressing on FP or platinum-based therapy were assigned to BSC plus either ramucirumab or placebo (20). OS, which served as the primary endpoint, modestly improved in the ramucirumab arm (5.2 vs. 3.8 months; HR 0.77, P=0.047). In addition, PFS (2.1 vs. 1.3 months; HR 0.48, P<0.05), and duration of disease control (4.2 vs. 2.9 months) improved. Symptom control and QoL were not significantly improved. In the second trial, patients (n=665) with disease progression on or within 4 months after platinum-based chemotherapy were assigned to paclitaxel alone or in combination with ramucirumab (21). OS (9.6 vs. 7.4 months; HR 0.807, P=0.017), PFS (HR 0.635, P<0.0001) and the disease control rate (DCR; 80 vs. 64%, P<0.0001) were significantly improved in the ramucirumab-containing arm at the cost of more grade 3 adverse events (AEs; neutropenia, hypertension, fatigue, anemia and abdominal pain). Given that ramucirumab alone confers an OS improvement of only 6 weeks, the combination with a taxane should be preferred in patients with PS 0–1. In a phase III study, patients (n=267) who failed ≥2 lines were assigned to receive apatinib, an oral VEGF-2 tyrosine kinase inhibitor (TKI), or placebo (22). Both OS (6.5 vs. 4.7 months; HR 0.71, P<0.016) and PFS (2.6 vs. 1.8 months; HR 0.44, P<0.001) were significantly improved in the experimental arm. However, apatinib resulted in a not negligible rate of grade 3–4 hand-foot syndrome (8.5%) with approximately half of the patients experiencing proteinuria (mainly grade 1–2) and 5.7% having grade 3–4 neutropenia. In addition, no significant improvement in QoL was observed in the apatinib arm. The small molecule inhibitor regorafenib inhibits endothelial cells by targeting VEGF-2 and TIE. Regorafenib was evaluated in a randomized phase II trial over BSC in 152 refractory patients up to a maximum of two lines (23). Regorafenib significantly prolonged PFS (2.6 vs. 0.9 months; HR 0.40, P<0.001) with a trend in OS, and the toxicity profile was consistent with that observed in other malignancies.
Human epidermal growth factor receptor 2 (HER2)
In first-line treatment, the addition of trastuzumab to platinum-based chemotherapy significantly prolongs OS in HER2+ metastatic gastric/esophagogastric junction cancer patients (9). Lapatinib is a dual HER2 and epidermal growth factor receptor TKI. In a phase III trial as a second-line therapy, no survival advantage was reported with lapatinib plus paclitaxel compared with paclitaxel alone in patients with HER2-amplified gastric cancer (24).
Mammalian target of rapamycin (mTOR)
In a phase III trial, everolimus was evaluated in patients who progressed after 1–2 lines of therapy, but no survival benefit emerged compared with the placebo arm (25).
Epidermal growth factor receptor (EGFR)
In phase III trials for previously untreated patients, the addition of cetuximab or panitumumab to platinum-based doublets did not improve survival compared with chemotherapy alone (26,27). In a phase III trial of second-line therapy, the TKI gefitinib was evaluated in patients with esophageal cancer, but no OS benefit was noted in the experimental arm compared with the placebo arm (28).
Poly ADP ribose polymerase (PARP)
PARP inhibition might be an effective strategy, particularly in cases exhibiting the coexistence of ataxia-telangiectasia mutated (ATM)-deficient cells and TP53 mutations (29). In a randomized phase II study of second-line therapy comparing paclitaxel either with olaparib or placebo (30), OS was significantly improved in both the overall (13.1 vs. 8.3 months; HR 0.56, P=0.005) and ATM low population (median OS not reached vs. 8.2 months; HR 0.35, P=0.002). Despite these promising data, the experimental arm in the follow-up phase III trial did not exhibit a significant improvement in OS in both the overall and ATM low populations (31).
Signal transducer and activator of transcription (STAT)
STAT3 is a transcription factor that when overactivated becomes an oncogenic signaling hub that promotes the stemness of cancer stem cells (CSCs), which is associated with resistance to conventional therapeutic agents. Napabucasin, an oral specific cancer CSCs inhibitor, was evaluated in combination with paclitaxel in a phase Ib/II study in pretreated patients. In total, 78% of these patients were previously administered ≥2 lines. In 20 patients who had not received a taxane, encouraging outcomes in terms of RR (31%), DCR (75%) and PFS (20.6 weeks) were reported (32). A phase III trial is ongoing comparing paclitaxel either plus napabucasin or placebo as a second-line therapy.
Phosphatidylinositol 3-kinase (PI3K)
Buparlisib (or BKM120) is an oral PI3K inhibitor evaluated in a phase II study in previously treated patients with platinum-based chemotherapy for esophageal squamous cell cancer. The reported DCR, PFS and OS were 51.2%, 2.0 and 9.0 months, respectively (33).
Immunotherapy
Cancer cells can evade detection and eradication by the immune system by reducing antigen expression, secreting immune-suppressive cytokines, or upregulating inhibitory signals (34). The modern immunotherapies block specific immune checkpoints such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), programmed cell death protein (PD-1) and its ligand (PD-L1). Nivolumab is the first agent demonstrating a survival benefit in pretreated gastric cancer patients. In the ONO-4538-12 phase III trial, patients refractory or intolerant to standard therapy were randomized to receive 3 mg/kg nivolumab every 2 weeks or placebo. PD-1 positivity was not required for study enrollment. Median OS, which served as the primary endpoint, was in favor of the nivolumab arm (5.32 vs. 4.14 months; HR 0.63, P<0.0001). In addition, PFS (1.61 vs. 1.45 months; HR 0.60, P<0.0001) and RR (11 vs. 0%, P<0.0001) were improved in the experimental arm. Nivolumab was well tolerated with a safety profile comparable to the placebo group (35). The anti-PD-1 agent pembrolizumab was evaluated in pretreated patients in 2 phase Ib trials with an RR ranging from 22% to 30% and grade 3–4 AEs that occurred in 13% and 17% of patients (36,37). Results consistent with pembrolizumab emerged in a recent large phase II trial (n=259) for patients treated with ≥2 lines. An overall RR of 11.2% was reported that was more pronounced in those exhibiting PD-L1+ tumors as assessed immunohistochemistry and defined as ≥1% tumor or stromal cells (38). Three phase III trials involving pembrolizumab in OGC are currently in progress; both trials are assessing first- and second-line therapies (39-41). In a phase Ib trial (42), the fully human anti-PD-L1 IgG1 antibody avelumab has demonstrated modest activity as first- (as a maintenance agent, RR 9%) and second-line (RR 10%) therapy. On the other hand, no activity has been observed with the anti-CTLA-4 antibody ipilimumab (43).
Pancreatic adenocarcinoma
Chemotherapy
Three systemic options can be considered in first-line treatment for patients with locally advanced unresectable or metastatic disease, i.e., gemcitabine alone or in combination with nab-paclitaxel (Gem-P) and the FOLFIRINOX regimen (44-46). As a consequence of better results obtained with combination regimens, approximately 40% of patients are considered for second-line treatments (47) given that a standard sequence is not established. In patients who progressed on gemcitabine, oxaliplatin-based regimens have been evaluated in 2 phase III trials. The CONKO-003 trial evaluated oxaliplatin added to 5-FU in the so-called OFF regimen in 160 patients (48). OS significantly improved in the oxaliplatin-containing arm compared with 5-FU alone (5.9 vs. 3.3 months; HR 0.66, P=0.010). The AEs were similar between the two groups except for increased neurotoxicity in the OFF arm (38.2% vs. 7.1%, P<0.001). In contrast, disappointing results were noted in the PANCREOX trial, in which patients (n=108) who progressed within 4 weeks of randomization either during or following prior gemcitabine were assigned to receive modified FOLFOX6 or infusional 5FU/LV (49). OS was reduced in the experimental arm with a surprisingly high outcome in the reference arm (6.1 vs. 9.9 months; P=0.02). In addition, more grade 3–4 AEs occurred in the FOLFOX6 arm (63% vs. 11%). The conflicting results of these two trials can be possibly explained by the reduced dose of oxaliplatin in the OFF regimen resulting in enhanced tolerability, and different eligibility criteria at progression status. Of note, the PANCREOX trial was closed prematurely due to slow accrual. A new formulation of irinotecan encapsulated into liposome-based nanoparticles was evaluated in a phase III trial in patients (n=417) who failed gemcitabine-containing therapy (50). Patients were assigned either to nanoliposomal irinotecan monotherapy, 5FU/folinic acid or the combination of both. Median OS was 6.1 months for nanoliposomal irinotecan monotherapy plus 5FU/LV and 4.2 months for 5FU/LV (HR 0.67, P=0.012). No significant difference in OS was noted between nanoliposomal irinotecan monotherapy and 5FU/LV. Nevertheless, the significant incidence of grade 3–4 AEs (diarrhea 13%, vomiting 11%, fatigue 14%, neutropenia 27%) in the experimental arm warrants caution in the systematic use of this regimen that is approved by the FDA. Indeed, ECOG PS 0–1, a relatively favorable comorbidity profile, an adequate supportive medical therapy and a port device are essential conditions for the application of this regimen. The optimal treatment for patients who progress on FOLFIRINOX is not established. In the PRODIGE4/ACCORD1 trial, approximately 50% of patients underwent second-line therapy. In those treated with FOLFIRINOX, gemcitabine was more often used as a single agent (82.5%) or in combination (12.5%), whereas FOLFOX (49.4%) or gemcitabine plus oxaliplatin (17.6%) were chosen in patients assigned to the gemcitabine arm. In a prospective multicenter cohort of 57 patients, encouraging PFS and OS (5.1 and 8.8 months, respectively) were reported using second-line Gem-P (51). This combination appeared to provide some clinical activity in retrospective single institution experiences (52,53). No standard options are available after failure of the Gem-P regimen. In the MPACT trial, 77% of patients received a FP-based regimen (54). The median OS for patients treated with a FP-containing second-line treatment after Gem-P was 13.5 months (vs. 9.5 for those treated with gemcitabine alone, P=0.012). Finally, some phase II trials evaluating single agents (i.e., docetaxel, paclitaxel or irinotecan) or combinations (i.e., oxaliplatin either plus raltitrexed or gemcitabine, FOLFIRI, XELOX or FOLFIRINOX) have reported OS values ranging from 4 to 8.5 months (55-63).
Targeted agents
With the exception of erlotinib, which confers a clinically negligible benefit when added to gemcitabine (64), first-line efforts to integrate a number of targeted agents have been disappointing. By inhibiting inflammation-promoted cancer progression, the JAK-STAT inhibitor ruxolitinib achieved promising outcomes in preclinical and phase II trials (65), but the subsequent phase III trial was terminated prematurely after demonstration of insufficient efficacy at a planned interim analysis of the JANUS1 trial. Additional strategies involved inhibitors of EGFR, HER2, IGF-1, VEGF, NOTCH, WNT and farnesyl-transferase pathways mainly in combination with gemcitabine (66). Early trials indicate that patients carrying BRCA mutations may benefit from platinum agents and PARP inhibitors. Promising activity of olaparib has been reported in a variety of different tumors associated with germline BRCA1/2 mutations (67), including PC (RR 21%, PFS 4.6 months and OS 9.8 months). A phase III trial of olaparib maintenance is currently enrolling patients with germline BRCA mutations who have not progressed on first-line platinum chemotherapy (68). Hyaluronidase over-accumulation in the extracellular matrix of many solid tumors is associated with tumor progression and poor prognosis (69). PEGPH20 has been developed to deplete tumor-associated hyaluronan in the extracellular matrix. In a phase Ib trial, twenty-eight patients were treated with escalating intravenous doses of PEGPH20 plus gemcitabine. Overall, PFS and OS were 5.0 months and 6.6 months, respectively. In 17 patients evaluated for pretreatment tissue hyaluronan levels, encouraging PFS and OS rates were 7.2 and 13.0 months, respectively, in those with high hyaluronan levels (70). A phase III trial of Gem-P +/− PEGPH20 is currently recruiting previously untreated patients who overexpress hyaluronan as assessed by immunohistochemistry (71). Abemaciclib is a selective ATP-competitive inhibitor of CDK4 and CDK6 kinases, preventing the phosphorylation and inactivation of the Rb tumor suppressor protein and subsequently inducing G1 cell cycle arrest and inhibition of cell proliferation. This compound is under evaluation in a randomized phase II trial as a single agent or in combination with either LY3023414 (PI3K/mTOR dual inhibitor) or galunisertib (TGF-βR1 inhibitor) versus chemotherapy in previously treated patients with metastatic disease (72). The vitamin D receptor is expressed in stroma from PC, and calcipotriol significantly reduces markers of inflammation and fibrosis in both in pancreatitis and the tumor stroma. Interestingly, targeting the vitamin D receptor leads to transcriptional reprogramming of pancreatic cancer stroma and improves the response to gemcitabine, which might have implications for therapeutic purposes (73).
Immunotherapy
Two vaccines have been evaluated in patients with advanced disease, namely, GV1001 (phase III as first-line therapy in combination with chemotherapy vs. chemotherapy alone) and GVAX (phase IIB after 2 prior lines in a 3-arm study in combination with the live-attenuated Listeria monocytogenes vaccine CRS-207, vs. either CRS-207 alone or chemotherapy). Unfortunately, in both trials the vaccine-containing arms failed to improve OS compared to chemotherapy alone (74,75). Other vaccines have been tested in randomized phase II trials in combination with gemcitabine, in particular the Wilms’ tumor (WT1) vaccine and IMM-101, a heat-killed Mycobacterium obuense (76,77). In the first trial, WT1 plus gemcitabine resulted in superior PFS compared with gemcitabine (133 vs. 76 days; HR 0.48, P=0.008). In the second trial, IMM-101 plus gemcitabine correlated with improved OS in a preplanned subgroup of metastatic patients (7.0 vs. 4.4 months; HR 0.54, P=0.01) compared with gemcitabine. Other therapeutic strategies in development include checkpoint inhibition combined with vaccines or combined immune checkpoint blockade.
Hepatobiliary cancers
Hepatocellular cancer (HCC)
HCC usually typically develops from a background of chronic liver diseases. Hepatitis B and C viruses, alcohol consumption, and non-alcoholic steatohepatitis represent frequent predisposing etiologies. The TKI sorafenib is the first agent that produced a survival benefit reported in two phase III trials over placebo (78,79). After sorafenib, a number of phase III trials evaluating other targeted agents (namely, brivanib, everolimus and ramucirumab) in first- and second-line settings have failed to improve OS as a consequence of marginal antitumor efficacy in this disease, risk of toxicity related to underlying liver dysfunction, lack of understanding of critical drivers of tumor progression/dissemination, imbalances in disease status (liver-only vs. metastatic), and different patient characteristics according to etiology of cirrhosis, Child-Pugh class and ethnicity (80).
Targeted agents
Encouraging results of second-line treatment emerged from the phase III RESORCE trial, in which 573 patients who failed sorafenib (given at ≥400 mg/day for ≥20 of last 28 days of treatment) were assigned to BSC plus either regorafenib or placebo (81). The majority of patients were Child-Pugh class A (98%). Approximately one-third of patients had macrovascular invasion, 70% had extrahepatic disease and 75% were cirrhotic. Regorafenib improved OS compared with BSC alone (10.6 vs. 7.8 months; HR 0.60, P<0.0001). The most relevant grade 3–4 AEs included hypertension (15%), hand-foot syndrome (13%), fatigue (9%), and diarrhea (3%). Extended knowledge is required in order to better clarify whether this agent can be used in patients who could not tolerate sorafenib and the optimal dose in this setting of patients at increased risk for toxicity. Cabozantinib blocks MET, RET and VEGF-2 receptors. This compound has been evaluated within a randomized placebo-controlled phase II trial in patients with Child-Pugh A class and ≤1 prior systemic therapy (82). A non-significant trend for PFS was observed in the experimental arm (2.5 vs. 1.4 months in the placebo arm). PFS and OS calculated from day 1 in all patients were 5.2 and 11.5 months, respectively. The phase III CELESTIAL trial comparing cabozantinib vs. placebo is ongoing in patients who progressed on sorafenib (83). Other potential therapeutic targets are the include EGFR/RAS/MAPK, IGF, PI13K/Akt/mTOR, Wnt-β-catenin, hedgehog, apoptotic, c-MET and antiangiogenic signaling pathways. The anti-angiogenic ramucirumab and c-MET inhibitor tivantinib, which were both evaluated as second-line therapies in 2 phase III trials, did not meet the primary endpoint of improving OS compared with placebo (84,85).
Immunotherapy
Immunological mechanisms are also assumed to play a crucial role in HCC proliferation (86). The rationale to target immune checkpoints is based on evidence that HCC may evade the immune system by expressing PD-1, CTLA-4, TIM-3, LAG-3 and others. Preclinical and clinical studies have demonstrated the potential benefit of modulating immunogenicity, and relevant approaches are currently being tested. Tremelimumab, an anti-CTLA-4 antibody, was evaluated in a pilot study involving 20 patients with HCC and chronic HCV infection. Of 17 assessable patients, the RR, DCR and time to progression were 17.6%, 76.4% and 6.5 months, respectively. In addition, a significant reduction of viral load was observed, and no major safety issues emerged (87). The results of a phase I/II study with the anti-PD-1 nivolumab were recently published. Patients (n=262) with advanced HCC and Child-Pugh score ≤7 who previously failed, refused or were intolerant to sorafenib were enrolled in three parallel cohorts based on the underlying disease etiology (no active hepatitis virus infection, HBV-infected, and HCV-infected). The reported RR was 20% in patients treated with 3 mg/kg nivolumab in the dose-expansion phase, and grade 3/4 AEs occurred in 25% of patients (88). Based on these data, nivolumab is currently being evaluated as a first-line agent compared with sorafenib in a phase III study (89). In addition, vaccines have been tested in clinical trials. The oncolytic vaccine virus Pexa-Vec delivered by intratumoral injection was evaluated in a randomized dose-finding phase II trial in patients with advanced disease (90). OS survival was significantly improved in the high-dose arm compared with the low-dose arm (14.1 vs. 6.7 months; HR 0.39, P=0.02) with an acceptable toxicity profile. Encouraging results from a phase II trial have been reported with another oral vaccine, hepcortespenlisimut-L. A drop of alpha fetoprotein was observed in 66.7% of patients, and OS was not reported given that 90.7% of patients were alive after a median follow-up of 12 months (91). Both vaccines are currently under evaluation in phase III trials (92).
Biliary tract cancer (BTC)
Chemotherapy
As first-line therapies, various non-randomized phase II trials evaluating gemcitabine and platinum-based regimens been published, with reported OS ranging from 8 to 15 months (93). In the large phase III ABC-02 trial, previously untreated patients (n=410) with intra- or extrahepatic cholangiocarcinoma, gallbladder cancer or ampullary cancer were randomized to receive either cisplatin plus gemcitabine for 8 cycles or gemcitabine alone. Median OS (11.7 vs. 8.1 months; HR 0.64, P<0.001), PFS (8.0 vs. 5.0 months, P<0.001) and DCR (81.4% vs. 71.8%, P=0.049) were increased for the doublet (94). Based on these results, cisplatin plus gemcitabine is a recognized first-line standard of care in advanced BTC. Only limited data of second-line therapy are available, and no randomized trials comparing systemic therapy versus BSC are published. Accordingly, the choice of second-line regimen is currently empiric. Experiences with single-agent FP or in combination with oxaliplatin have been reported in patients who failed cisplatin plus gemcitabine, with RR ranging from 1% for FP alone to 8% to 22% for FP plus oxaliplatin (95,96).
Targeted agents
As first-line agents, targeted agents have been incorporated in phase II trials (either single arm or randomized), including bevacizumab (plus erlotinib) or erlotinib alone, cetuximab, or panitumumab (plus gemcitabine and oxaliplatin). Modest or even absent significant clinical activity was noted at the cost of relevant toxicities (97-99). In a meta-analysis of 6 trials involving 855 patients treated either with erlotinib, cetuximab, panitumumab, cediranib or sorafenib, no OS advantage was observed in the experimental arms despite enhanced or a trend for enhanced RR and PFS (100). As second-line agents, in a very small retrospective analysis of 13 patients refractory to GEMOX, FOLFIRI plus bevacizumab achieved a RR of 41% with a median PFS and OS of 7.6 and 14.2 months, respectively (101). In a phase II trial for both chemo-naive and pretreated (one prior line permitted) patients, some signals of activity have been observed with erlotinib given that 17% of patients were progression-free at 6 months (102). In a phase II study, patients (n=26) who failed ≥1 chemotherapy line and harbored fusions or other FGFR alterations were treated with BGJ398, a selective pan-FGFR inhibitor (103). Among 22 evaluable patients, three achieved partial response, and 15 had stable disease (including 10 experiencing some tumor reduction) for an overall DCR of 82%. In a phase II study with selumetinib, an inhibitor of MEK1/2, 12% of patients experienced a response with PFS and OS of 3.7 of 9.8 months, respectively (104). The TKI regorafenib has been evaluated as a second-line therapy in a phase II trial, and data have been recently presented (105). The primary endpoint was met with a reported PFS of 3.55 months. OS was 5.55 months, and grade 3–4 AEs occurred in 40.5% of cases. Phase I trials targeting isocitrate dehydrogenase in enriched populations, including BTC patients, are underway. CAP7.1 is a compound that releases etoposide in the presence of carboxylesterases, leading to increased intra-tumor etoposide concentration. Patients with BTC were assigned to either CAP7.1 or BSC in a randomized phase II trial allowing crossover at progression (106). Some antitumor activity emerged (DCR 59%, PFS 3.5 months in the experimental arm) with overall manageable toxicity (mainly hematological).
Miscellanea
In a retrospective study of 603 patients who failed gemcitabine and platinum therapy, second-line therapy (including irinotecan- or oxaliplatin-based chemotherapy, single agent FP, sunitinib or other regimens) was administered to 196 patients. Among 186 evaluable patients, reported PFS and OS were 3.2 and 6.7 months, respectively. In addition, FP-based doublets were not superior to FP alone (107). Similar survival results were reported in another retrospective analysis of 174 patients, achieving a PFS and OS of 3 and 6.6 months, respectively. These results have been substantiated in a pooled analysis of 5 additional studies (n=499), reporting a median OS of 6.3 months (108). Both studies identified potentially favorable prognostic factors, i.e., good PS, low Ca19.9 levels, absence of distant metastases and duration of disease control on first-line therapy. Once again, in a systematic review including 14 phase II trials, 9 retrospective analyses and 2 case reports (n=761), the mean OS was 7.2 months (109).
Immunotherapy
Chronic inflammation plays a crucial role in the development of BTC. Cholelithiasis, parasites, HCV infection, primary biliary cirrhosis and sclerosing cholangitis are well known risk factors. This knowledge represents the platform to implement immunotherapeutic strategies in BTC, and a number of clinical trials involving either peptide-based vaccines or dendritic cell-based vaccines have been conducted. Promising results have been reported that warrant further confirmation (110). Single agent pembrolizumab has been evaluated in patients with PD-L1-positive gallbladder or biliary tree adenocarcinomas as a part of the KEYNOTE-028 phase Ib trial (111). Twenty-four out 37 identified patients were enrolled, and all received at least 1 prior line of therapy (38% of them ≥3 regimens). The RR was 17.4%, and pembrolizumab was generally well tolerated (grade 3 AEs reported in 17% of patients).
Conclusions
Advanced UGC are highly fatal malignancies characterized by marked genetic complexity. The development of second-line systemic therapies for these patients has been met with only few successes over recent decades (Table 1). Based on better understanding of molecular drivers and the advent of new targeted agents, UGC are increasingly entering into the era of personalized medicine. Research on immunotherapies in these malignancies is vibrant, aiming to identify predictive and prognostic biomarkers to define subgroups of patients who are most likely to benefit from these agents. In this regard, an important achievement has been the proof of clinical activity of PD-1 blockade in MSI-high colorectal and non-colorectal cancers, which lead FDA to approve approval of pembrolizumab for all MSI-H/MMR-deficient cancers (112). Of interest, a high tumor mutational burden seems to predict favorable outcome to PD-1/PD-L1 blockade across various cancers (113). In addition, other biomarkers, including serum proteins, tumor-specific receptor expression patterns, factors in the tumor microenvironment, circulating immune and tumor cells, and host genomic factors, are potential candidates in predicting response to immunotherapy (114). Finally, distinct molecular subtypes of gastric, esophageal cancer and PC have been identified, providing a guide to targeted strategies that should be evaluated in clinical trials (115-117). Global collaborative efforts are essential to give a decisive boost to further improve survival in these patients.
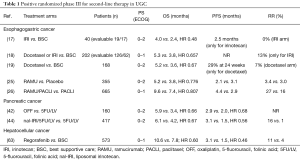
Full table
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Sargent DJ, Köhne CH, Sanoff HK, et al. Pooled safety and efficacy analysis examining the effect of performance status on outcomes in nine first-line treatment trials using individual data from patients with metastatic colorectal cancer. J Clin Oncol 2009;27:1948-55. [Crossref] [PubMed]
- Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457-65. [Crossref] [PubMed]
- Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 2005;55:241-52. [Crossref] [PubMed]
- Schrag D, Archer L, Wang X, et al. A patterns-of-care study of post-progression treatment (Rx) among patients (pts) with advanced pancreas cancer (APC) after gemcitabine therapy on Cancer and Leukemia Group B (CALGB) study #80303. J Clin Oncol 2007;25 Suppl 18:abstr 4524.
- Ji SH, Lim DH, Yi SY, et al. A retrospective analysis of second-line chemotherapy in patients with advanced gastric cancer. BMC Cancer 2009;9:110. [Crossref] [PubMed]
- Kim MJ, Oh DY, Lee SH, et al. Gemcitabine-based versus fluoropyrimidine-based chemotherapy with or without platinum in unresectable biliary tract cancer: a retrospective study. BMC Cancer 2008;8:374. [Crossref] [PubMed]
- Ter Veer E, Haj Mohammad N, van Valkenhoef G, et al. The efficacy and safety of first-line chemotherapy in advanced esophagogastric cancer: a network meta-analysis. J Natl Cancer Inst 2016;108. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer–a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47:2306-14. [Crossref] [PubMed]
- Kang JH, Lee SI, Lim DH, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30:1513-8. [Crossref] [PubMed]
- Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol 2014;15:78-86. [Crossref] [PubMed]
- Janowitz T, Thuss-Patience P, Marshall A, et al. Chemotherapy vs supportive care alone for relapsed gastric, gastroesophageal junction, and oesophageal adenocarcinoma: a meta-analysis of patient-level data. Br J Cancer 2016;114:381-7. [Crossref] [PubMed]
- Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31:4438-44. [Crossref] [PubMed]
- Higuchi K, Tanabe S, Shimada K, et al. Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer 2014;50:1437-45. [Crossref] [PubMed]
- Nishikawa K, Fujitani K, Inagaki H, et al. Randomised phase III trial of second-line irinotecan plus cisplatin versus irinotecan alone in patients with advanced gastric cancer refractory to S-1 monotherapy: TRICS trial. Eur J Cancer 2015;51:808-16. [Crossref] [PubMed]
- Koeda K, Shitara K, Takashima A, et al. ABSOLUTE: A phase 3 trial of nanoparticle albumin-bound paclitaxel (nab-PTX) versus solvent-based paclitaxel (sb-PTX) in patients with pre-treated advanced gastric cancer (AGC)-Efficacy and QOL results. J Clin Oncol 2017;35:abstr 4010.
- Schmalenberg H, Pauligk C, Zander T, et al. A multicentre, phase II study with cabazitaxel in previously treated patients with advanced or metastatic adenocarcinoma of the oesophagogastric junction and stomach (CABAGAST). J Clin Oncol 2017;35:abstr 4054.
- Ohtsu A, Shah MA, Van Cutsem E, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol 2011;29:3968-76. [Crossref] [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [Crossref] [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [Crossref] [PubMed]
- Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448-54. [Crossref] [PubMed]
- Pavlakis N, Sjoquist KM, Martin AJ, et al. Regorafenib for treatment of advanced gastric cancer (INTEGRATE): a multinational placebo-controlled phase II trial. J Clin Oncol 2016;34:2728-35. [Crossref] [PubMed]
- Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN--a randomized, phase III study. J Clin Oncol 2014;32:2039-49. [Crossref] [PubMed]
- Ohtsu A, Ajani JA, Bai YX, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, phase III GRANITE-1 study. J Clin Oncol 2013;31:3935-43. [Crossref] [PubMed]
- Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:490-9. [Crossref] [PubMed]
- Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013;14:481-9. [Crossref] [PubMed]
- Dutton SJ, Ferry DR, Blazeby JM, et al. Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol 2014;15:894-904. [Crossref] [PubMed]
- Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 2008;9:759-69. [Crossref] [PubMed]
- Bang YJ, Im SA, Lee KW, et al. Randomized, double-blind phase II trial with prospective classification by ATM protein level to evaluate the efficacy and tolerability of olaparib plus paclitaxel in patients with recurrent or metastatic gastric cancer. J Clin Oncol 2015;33:3858-65. [Crossref] [PubMed]
- Bang Y, Boku N, Chin K, et al. Olaparib in combination with paclitaxel in patients with advanced gastric cancer who have progressed following first-line therapy: phase III GOLD study. ESMO 2016. Abstract LBA25.
- Becerra C, Stephenson J, Jonker DJ, et al. Phase Ib/II study of cancer stem cell (CSC) inhibitor BBI608 combined with paclitaxel in advanced gastric and gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol 2015;33:abstr 4069.
- Kato K, Doi T, Kojima T, et al. Phase II study of BKM120 in patients with advanced esophageal squamous cell carcinoma (EPOC1303). J Clin Oncol 2017;35:abstr 4069.
- Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321-30. [Crossref] [PubMed]
- Kang YK, Satoh T, Ryu MH, et al. Nivolumab (ONO-4538/BMS-936558) as salvage treatment after second or later-line chemotherapy for advanced gastric or gastro-esophageal junction cancer (AGC): a double-blinded, randomized, phase III trial. J Clin Oncol. 2017;35:abstr 2.
- Muro K, Chung HC, Shankaran V, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol 2016;17:717-26. [Crossref] [PubMed]
- Doi T, Piha-Paul SA, Jalal SA, et al. Updated results for the advanced esophageal carcinoma cohort of the phase 1b KEYNOTE-028 study of pembrolizumab. J Clin Oncol 2016;34:abstr 4046.
- Fuchs CS, Doi T, Woo-Jun Jang R, et al. KEYNOTE-059 cohort 1: Efficacy and safety of pembrolizumab (pembro) monotherapy in patients with previously treated advanced gastric cancer. J Clin Oncol 2017;35:abstr 4003.
- Tabernero J, Bang YJ, Fuchs CS, et al. KEYNOTE-062: Phase III study of pembrolizumab (MK-3475) alone or in combination with chemotherapy versus chemotherapy alone as first-line therapy for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma. J Clin Oncol 2016;34:abstr TPS185.
- Ohtsu A, Tabernero J, Bang YJ, et al. Pembrolizumab (MK-3475) versus paclitaxel as second-line therapy for advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma: phase 3 KEYNOTE-061 study. J Clin Oncol 2016;34:abstr TPS183.
- Doi T, Bennouna J, Shen L, et al. KEYNOTE-181: Phase 3, open-label study of second-line pembrolizumab vs single-agent chemotherapy in patients with advanced/metastatic esophageal adenocarcinoma. J Clin Oncol 2016;34:abstr TPS4140.
- Chung HC, Arkenau HT, Wyrwicz L, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced gastric or gastroesophageal junction cancer from JAVELIN solid tumor phase Ib trial: analysis of safety and clinical activity. J Clin Oncol 2016;34:abstr 4009.
- Moehler MH, Cho JY, Kim YH, et al. A randomized, open-label, two-arm phase II trial comparing the efficacy of sequential ipilimumab (ipi) versus best supportive care (BSC) following first-line (1 L) chemotherapy in patients with unresectable, locally advanced/metastatic (A/M) gastric or gastro-esophageal junction (G/GEJ) cancer. J Clin Oncol 2016;34:abstr 4011.
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, al . Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Smyth EN, Bapat B, Ball DE, et al. Metastatic pancreatic adenocarcinoma treatment patterns, health care resource use, and outcomes in France and the United Kingdom between 2009 and 2012: a retrospective study. Clin Ther 2015;37:1301-16. [Crossref] [PubMed]
- Oettle H, Riess H, Stieler JM, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol 2014;32:2423-9. [Crossref] [PubMed]
- Gill S, Ko YJ, Cripps C, et al. PANCREOX: A randomized phase III study of 5-Fluorouracil/Leucovorin with or without oxaliplatin for second-Line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J Clin Oncol 2016;34:3914-20. [Crossref] [PubMed]
- Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinicacid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global,randomised, open-label, phase 3 trial. Lancet 2016;387:545-57. [Crossref] [PubMed]
- Portal A, Pernot S, Tougeron D, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer 2015;113:989-95. [Crossref] [PubMed]
- Zhang Y, Hochster H, Stein S, et al. Gemcitabine plus nab-paclitaxel for advanced pancreatic cancer after first-line FOLFIRINOX: single institution retrospective review of efficacy and toxicity. Exp Hematol Oncol 2015;4:29. [Crossref] [PubMed]
- Bertocchi P, Abeni C, Meriggi F, et al. Gemcitabine plus Nab-Paclitaxel as second-line and beyond treatment for metastatic pancreatic cancer: a single institution retrospective analysis. Rev Recent Clin Trials 2015;10:142-5. [Crossref] [PubMed]
- Chiorean EG, Von Hoff DD, Tabernero J, et al. Second-line therapy after nab-paclitaxel plus gemcitabine or after gemcitabine for patients with metastatic pancreatic cancer. Br J Cancer 2016;115:188-94. [Crossref] [PubMed]
- Reni M, Pasetto L, Aprile G, et al. Raltitrexed-eloxatin salvage chemotherapy in gemcitabine-resistant metastatic pancreatic cancer. Br J Cancer 2006;94:785-91. [Crossref] [PubMed]
- Demols A, Peeters M, Polus M, et al. Gemcitabine and oxaliplatin (GEMOX) in gemcitabine refractory advanced pancreatic adenocarcinoma: a phase II study. Br J Cancer 2006;94:481-5. [Crossref] [PubMed]
- Saif MW, Syrigos K, Penney R, et al. Docetaxel second-line therapy in patients with advanced pancreatic cancer: a retrospective study. Anticancer Res 2010;30:2905-9. [PubMed]
- Oettle H, Arnold D, Esser M, et al. Paclitaxel as weekly second-line therapy in patients with advanced pancreatic carcinoma. Anticancer Drugs 2000;11:635-8. [Crossref] [PubMed]
- Yi SY, Park YS, Kim HS, et al. Irinotecan monotherapy as second-line treatment in advanced pancreatic cancer. Cancer Chemother Pharmacol 2009;63:1141-5. [Crossref] [PubMed]
- Zaniboni A, Aitini E, Barni S, et al. FOLFIRI as second-line chemotherapy for advanced pancreatic cancer: a GISCAD multicenter phase II study. Cancer Chemother Pharmacol 2012;69:1641-5. [Crossref] [PubMed]
- Xiong HQ, Varadhachary GR, Blais JC, et al. Phase 2 trial of oxaliplatin plus capecitabine (XELOX) as second-line therapy for patients with advanced pancreatic cancer. Cancer 2008;113:2046-52. [Crossref] [PubMed]
- Lee MG, Lee SH, Lee SJ, et al. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with advanced pancreatic cancer who have progressed on gemcitabine-based therapy. Chemotherapy 2013;59:273-9. [Crossref] [PubMed]
- Assaf E, Verlinde-Carvalho M, Delbaldo C, et al. 5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology 2011;80:301-6. [Crossref] [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [Crossref] [PubMed]
- Hurwitz HI, Uppal N, Wagner SA, et al. Randomized, Double-Blind, Phase II Study of Ruxolitinib or Placebo in Combination With Capecitabine in Patients With Metastatic Pancreatic Cancer for Whom Therapy With Gemcitabine Has Failed. J Clin Oncol 2015;33:4039-47. [Crossref] [PubMed]
- Andrikou K, Peterle C, Pipitone S, et al. Emerging antibodies for the treatment of pancreatic cancer. Expert Opin Emerg Drugs 2017;22:39-51. [Crossref] [PubMed]
- Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 2015;33:244-50. [Crossref] [PubMed]
- Kindler HL, Locker GY, Mann H, et al. POLO: A randomized phase III trial of olaparib tablets in patients with metastatic pancreatic cancer (mPC) and a germline BRCA1/2mutation (gBRCAm) who have not progressed following first-line chemotherapy. J Clin Oncol 2015;33:abstr TPS4149.
- Provenzano PP, Cuevas C, Chang AE, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 2012;21:418-29. [Crossref] [PubMed]
- Hingorani SR, Harris WP, Beck JT, et al. Phase Ib study of PEGylated recombinant human hyaluronidase and gemcitabine in patients with advanced pancreatic cancer. Clin Cancer Res 2016;22:2848-54. [Crossref] [PubMed]
- ClinicalTrials.gov. A Study of PEGylated Recombinant Human Hyaluronidase in Combination With Nab-Paclitaxel Plus Gemcitabine Compared With Placebo Plus Nab-Paclitaxel and Gemcitabine in Participants With Hyaluronan-High Stage IV Previously Untreated Pancreatic Ductal Adenocarcinoma. Available online: NCT02715804http://clinicaltrials.gov/ct2/show/NCT02715804?term=
- Chiorean EG, Hochster HS, Nanda S, et al. A phase II study of abemaciclib as a monotherapy and in combination with other agents in patients with previously treated metastatic pancreatic ductal adenocarcinoma (PDAC). J Clin Oncol 2017;35:abstr TPS4150.
- Sherman MH, Yu RT, Engle DD, et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014;159:80-93. [Crossref] [PubMed]
- Middleton G, Silcocks P, Cox T, et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol 2014;15:829-40. [Crossref] [PubMed]
- Le DT, Ko AH. Wainberg ZA at al. Results from a phase 2b, randomized, multicenter study of GVAX pancreas and CRS-207 compared to chemotherapy in adults with previously-treated metastatic pancreatic adenocarcinoma (ECLIPSE Study). J Clin Oncol 2017.35. abstract 345.
- Nishida S, Ishikawa T, Kokura S, et al. Randomized phase II study of WT1 peptide vaccine plus gemcitabine for advanced pancreatic ductal adenocarcinoma (PDAC): clinical efficacy and immune response. J Clin Oncol 2016;34:abstr 3085.
- Dalgleish AG, Stebbing J, Douglas JA, et al. Randomised, open-label, phase II study of gemcitabine with and without IMM-101 for advanced pancreatic cancer. Br J Cancer 2016;115:789-96. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Torrecilla S, Llovet JM. New molecular therapies for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2015;39 Suppl 1:S80-5. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Kelley RK, Verslype C, Cohn AL, et al. Cabozantinib in hepatocellular carcinoma: results of a phase 2 placebo-controlled randomized discontinuation study. Ann Oncol 2017;28:528-34. [Crossref] [PubMed]
- Abou-Alfa GK, Cheng Al, Meyer T, et al. Phase 3 randomized, double-blind, controlled study of cabozantinib (XL184) versus placebo in subjects with hepatocellular carcinoma who have received prior sorafenib (CELESTIAL; NCT01908426). J Clin Oncol 2014;32:abstr TPS4150.
- Zhu AX, Park JO, Ryoo B, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-70. [Crossref] [PubMed]
- Rimassa L, Assenat E, Peck-Radosavljevic M, et al. Second-line tivantinib (ARQ 197) vs placebo in patients (Pts) with MET-high hepatocellular carcinoma (HCC): results of the METIV-HCC phase III trial. J Clin Oncol 2017;35:abstr 4000.
- Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, et al. The yin and yang of evasion and immune activation in HCC. J Hepatol 2015;62:1420-9. [Crossref] [PubMed]
- Sangro B, Gomez-Martin C, de la Mata M, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J Hepatol 2013;59:81-8. [Crossref] [PubMed]
- El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492-502. [Crossref] [PubMed]
- Sangro B, Park J-W, Dela Cruz CM, et al. A randomized, multicenter, phase 3 study of nivolumab vs sorafenib as first-line treatment in patients (pts) with advanced hepatocellular carcinoma (HCC): CheckMate-459. J Clin Oncol 2016;34:abstr TPS4147.
- Heo J, Reid T, Ruo L, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 2013;19:329-36. [Crossref] [PubMed]
- Tarakanovskaya MG, Chinburen J, Batchuluun P, et al. Open-label Phase II clinical trial in 75 patients with advanced hepatocellular carcinoma receiving daily dose of tableted liver cancer vaccine, hepcortespenlisimut-L. J Hepatocell Carcinoma 2017;4:59-69. [Crossref] [PubMed]
- Longo V, Gnoni A, Casadei Gardini A, et al. Immunotherapeutic approaches for hepatocellular carcinoma. Oncotarget 2017;8:33897-910. [Crossref] [PubMed]
- Schweitzer N, Vogel A. Systemic therapy of cholangiocarcinoma: from chemotherapy to targeted therapies. Best Pract Res Clin Gastroenterol 2015;29:345-53. [Crossref] [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [Crossref] [PubMed]
- Kim BJ, Yoo C, Kim KP, et al. Efficacy of fluoropyrimidine-based chemotherapy in patients with advanced biliary tract cancer after failure of gemcitabine plus cisplatin: retrospective analysis of 321 patients. Br J Cancer 2017;116:561-7. [Crossref] [PubMed]
- He S, Shen J, Sun X, et al. A phase II FOLFOX-4 regimen as second-line treatment in advanced biliary tract cancer refractory to gemcitabine/cisplatin. J Chemother 2014;26:243-7. [Crossref] [PubMed]
- Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 2010;28:3491-7. [Crossref] [PubMed]
- Malka D, Cervera P, Foulon S, et al. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol 2014;15:819-28. [Crossref] [PubMed]
- Leone F, Marino D, Cereda S, et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer 2016;122:574-81. [Crossref] [PubMed]
- Zhao S, Miao Y, Wang R, et al. Efficacy and toxicities of adding molecular targeted agents to first-line chemotherapy in the treatment of advanced biliary tract cancer: a systematic review and meta-analysis. Onco Targets Ther 2016;9:6695-700. [Crossref] [PubMed]
- Guion-Dusserre JF, Lorgis V, Vincent J, et al. FOLFIRI plus bevacizumab as a second-line therapy for metastatic intrahepatic cholangiocarcinoma. World J Gastroenterol 2015;21:2096-101. [Crossref] [PubMed]
- Philip PA, Mahoney MR, Allmer C, et al. Phase II study of erlotinib in patients with advanced biliary cancer. J Clin Oncol 2006;24:3069-74. [Crossref] [PubMed]
- Javle MM, Shroff RT, Zhu A, et al. A phase 2 study of BGJ398 in patients (pts) with advanced or metastatic FGFR-altered cholangiocarcinoma (CCA) who failed or are intolerant to platinum-based chemotherapy. J Clin Oncol 2016;34:abstr 335.
- Bekaii-Saab T, Phelps MA, Li X, et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 2011;29:2357-63. [Crossref] [PubMed]
- Sun W, Normolle DP, Bahary N, et al. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy refractory advanced and metastatic biliary adenocarcinoma/cholangiocarcinoma. J Clin Oncol 2017;35:abstr 4081.
- Pape UF, Kasper S, Sinn M, et al. Randomized, multicenter phase II trial of CAP7.1 in patients with advanced biliary tract cancers. J Clin Oncol 2016;34:abstr 441.
- Brieau B, Dahan L, De Rycke Y, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: A large multicenter study by the Association des Gastro-Entérologues Oncologues. Cancer 2015;121:3290-7. [Crossref] [PubMed]
- Fornaro L, Vivaldi C, Cereda S, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res 2015;34:156. [Crossref] [PubMed]
- Lamarca A, Hubner RA, David Ryder W, et al. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol 2014;25:2328-38. [Crossref] [PubMed]
- Marks EI, Yee NS. Immunotherapeutic approaches in biliary tract carcinoma: Current status and emerging strategies. World J Gastrointest Oncol 2015;7:338-46. [Crossref] [PubMed]
- Bang YJ, Doi T, De Braud F, et al. Safety and efficacy of pembrolizumab (MK-3475) in patients (pts) with advanced biliary tract cancer: Interim results of KEYNOTE-028. Eur J Cancer 2015;51:abstr 525.
- Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598-608. [Crossref] [PubMed]
- Spencer KR, Wang J, Silk AW, et al. Biomarkers for immunotherapy: current developments and challenges. Am Soc Clin Oncol Educ Book 2016;35:e493-503. [Crossref] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202-9. [Crossref] [PubMed]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017;541:169-75. [Crossref] [PubMed]
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52. [Crossref] [PubMed]


