Laparoscopic rectal resection versus open rectal resection with minilaparotomy for invasive rectal cancer
Introduction
Since first described in 1991, laparoscopic surgery has been increasingly advocated as a safe and efficient technique for the treatment of colorectal cancer (1). In comparison with conventional open surgery, laparoscopic colorectal resection was shown to be associated with reduced blood loss, less postoperative pain and a shorter hospital stay (2-5). However, laparoscopic colorectal surgery requires special instruments and costly disposables. It is associated with a steep learning curve and longer operation time (6-8).
Although instrumentation, surgical skills and techniques in laparoscopic surgery have evolved, it is often necessary to extract the surgical specimen and perform the bowel anastomosis through a small skin incision. Recent studies reported that a minilaparotomy (incision ≤7 cm) was technically feasible and safe for colorectal cancer resections and was associated with a rapid postoperative recovery (9-12). In addition, this technique did not increase operating time and the learning curve was less steep as compared with laparoscopic colorectal surgery.
A study by Nakagoe et al. suggested that minilaparotomy technique for resection of rectal cancer was an attractive alternative in non-overweight patients (12). Very few studies have compared short and long term outcomes of minilaprotomy surgery for the treatment of rectal cancer (13). Hence, the oncological adequacy of minilaparotomy approach in rectal cancer remains to be determined. The aim of our study was to assess the long-term clinical and oncological outcome after laparoscopic and minilaparotomy surgery in patients with rectal cancer.
Patients and methods
Definition of minilaparotomy
The minilaparotomy approach for the resection of rectal cancer is defined as a resection performed through a skin incision ≤7 cm in length.
Patients
All patients with a rectal cancer with the edge ≤12 cm from the anal verge without other concurrent or previous malignant disease treated by minilaparotomy and laparoscopic surgery were compared retrospectively. Evaluation included physical examination, colonoscopy with biopsy, anorectal ultrasonography, pelvic magnetic resonance and thoracic and abdominal computed tomography (CT). The mobility and the location of the tumor from the anal verge were assessed by digital examination by the surgeon and radiological imaging. Patients were staged using the clinical tumor node metastasis (TNM) classification. Exclusion criteria were patients who refused to consent for the study, and patients with tumors infiltrating to adjacent organs (cT4). Patients who had associated gastrointestinal diseases that required additional extensive operative intervention or evaluation were excluded. Patients with evidence of synchronous metastatic disease were also excluded. The choice between minilaparotomy and laparoscopic surgery was based on a joint decision by the patients and doctors. This study was approved by our local research ethics committee. Written informed consent was obtained from all patients.
Preoperative preparation and neoadjuvant chemoradiotherapy
All patients had bowel preparations, including a fluid diet and administration of a polyethylene glycol electrolyte solution, one day before the operation unless there were contraindications against bowel preparation. Intravenous antibiotic prophylaxis was given on induction of anesthesia for the operation.
The basic indications for neoadjuvant chemoradiotherapy included rectal cancers (T3) and/or node-positive disease, lack of prior radiation therapy to the pelvis, and age <75 years. Neoadjuvant treatment with chemotherapy and radiation therapy was as follows: 45 Gy in five weeks with concomitant 5-fluorouracil. The operation was carried out six to eight weeks after the end of the neoadjuvant treatment.
Operation techniques
All operations were performed by the same surgical team, which included TZ, GZ, and ZL and all of whom had experience in minilaparotomy and laparoscopic approaches to rectal cancer.
All patients underwent TME with preservation of the hypogastric nerves. Abdominoperineal resection (APR) was performed when the tumor infiltrated the anal canal or when it was impossible to obtain a distal margin of more than 1 cm. For low anterior resection (LAR), stapled end-to-end colorectal anastomoses were constructed. The rectal resection via minilaparotomy approach started with a midline skin incision from the pubis towards the umbilicus less than or equal to 7 cm long (12) (Figures 1,2). In case a laparoscopic operation was performed, a five-port technique was used as described previously (14). Both approaches adhered to the principles of total mesorectal excision. Procedures were carried out using the medial-to-lateral approach. The root of the main mesenteric vascular pedicles was initially dissected with lymphadenectomy, and the mesentery and diseased segment of bowel were mobilized from the retroperitoneum.
Patients undergoing LAR received a 5 cm incision for the removal of the specimen and placement of the stapler head. For patients undergoing APR or coloanal anastomosis, specimens were removed through the perineum with no need for an abdominal incision. The protective colostomy was not performed in all patients. Splenic flexure mobilization was conducted when necessary in the laparoscopic approach, but was not performed in the minilaparotomy approach because of small incision. Conversion to open surgery was needed if the surgeon was unable to complete the laparoscopic resection.
Postoperative care
Patients in both groups were managed by the same postoperative protocol, which included removal of the nasogastric tube at the end of the operation and oral liquids on postoperative day 1. Oral diet was resumed once there were passage of flatus and return of bowel function clinically. Pethidine 1 mg/kg was administered parenterally every 4 h on demand. The patients were discharged when they were fully ambulatory, were passing stools and flatus, could drink and eat solid foods and had no postoperative discomfort. After laparoscopy and open surgery, stage III patients received postoperative adjuvant chemotherapy with 5-fluorouracil and leucovorin for six months.
Follow up
Operative time, blood loss, time to first bowel movement, time to normal diet, length of stay, pain score using visual analogue scale ranging from zero to ten, and complications were recorded. Postoperative complications were classified according to the Clavien-Dindo classification of surgical complications and the grades of complication were recorded. Bladder evacuation disorder was defined as urinary incontinence or incomplete evacuation necessitating catheterization >4 weeks after surgery. Postoperative sexual dysfunction was defined as new onset erectile and/or ejaculatory dysfunction in male patients and as impairment of vaginal lubrication in female patients. The data was collected using the European Organization for Research and Treatment of Cancer (EORTC) QLQ-CR38 questionnaire at 24 months after initial surgery. The costs of the two operations were estimated by summing up the market value of theater time, disposable instruments used, and hospitalization service charge.
After discharge, follow-up was arranged regularly for clinical examination and carcinoem-bryonic antigen (CEA) test at 3-month intervals in the first two years and at 6-month intervals thereafter. The ultrasonography or imaging was not routinely performed. This was only indicated when there was a clinical suspicion of disease recurrence or when CEA level increased over time.
Local recurrence of cancer was defined as the radiologically evidence of tumor recurrence and/or histologically proven tumor within the operation field. Local recurrence in combination with distant recurrence was also considered as a local recurrence event. Distant metastases were defined as any recurrence occurring outside the pelvis.
Pathological evaluation
The rectal specimen was examined in the operation room by the surgeon to assess the distal resection margin and was then sent fresh to the histopathological department, where it was pinned on a cork board. The surface of the mesorectum was inked before slicing to assess the circumferential resection margin. Microscopic assessment included tumour infiltration through the bowel wall (T), the presence of positive lymph nodes (N), and analysis of the distal and circumferential resection margins. The circumferential resection margin was considered to be positive if it was <1 mm.
Statistical analysis
Data were analyzed using the SPSS 16.0 software (SPSS, Chicago, IL, USA). The chi-square test was used for categorical variables. The Student t test or Mann-whitney U test were used for parametric and nonparametric continuous variables. Survival was calculated using the Kaplan-Meier method, and comparison between survival curves was performed using the log-rank test. Statistical significance was defined as P<0.05.
Results
Characteristics of patients
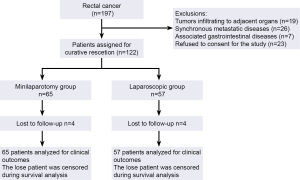
Between January 2005 and January 2008, 197 patients with rectal cancer were deemed eligible for participation in the study. Seventy five patients were excluded. The remaining 122 patients were allocated to rectal resection via a minilaparotomy (n=65) or via the laparoscopic approach (n=57). The consort flow chart is presented in Figure 3.
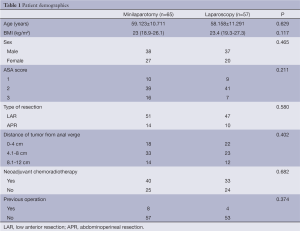
There were no differences between the two groups with regard to age, gender, ASA class, history of prior abdominal surgery and tumor location (Table 1).
Full table
Operative details
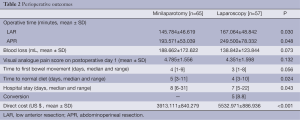
As shown in Table 2, operative time was longer in the laparoscopic group, but the time to resumption of normal diet was significantly shorter in the laparoscopic group as compared to the minilaparotomy group (median 4 vs. 5 days, P=0.024). Both groups were comparable for postoperative pain score. There were no differences between the two groups for the time to first bowel movement (median 3 vs. 4 days, P=0.056).Length of hospital stay was lower in the laparoscopic group (median 7 vs. 8 days, P=0.043).
Full table
The rate of conversion was 8.8% (5/57). The reasons for conversion were bleeding (n=1), pelvic adhesion (n=1) and difficulty in obtaining distal length to accomplish the anastomosis (n=3).The costs in the laparoscopic group were significantly higher than the minilaparotomy group (mean USD 5,532 vs. USD 3,913, P<0.001) (Table 2).
Mortality and morbidity
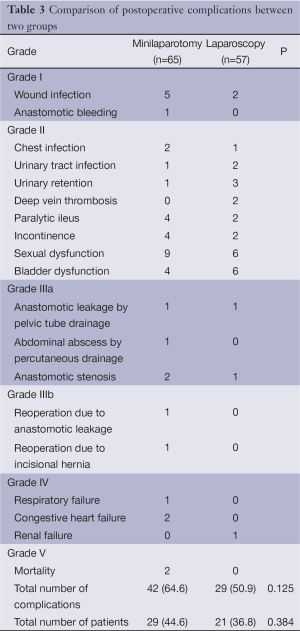
There were no postoperative mortality in the laparoscopic group, and two deaths occurred in the minilaparotomy group due to pulmonary embolism and myocardial infarction respectively. Twenty one patients had complications in the laparoscopic group (36.8%) and 29 patients had complications in the minilaparotomy group (44.6%). The total number of adverse events were 29 (50.9%) and 42 (64.6%), respectively (Table 3).In the minilaparotomy group, reoperation was required in two patients due to anastomotic leak (n=1) and incisional hernia (n=1).
Full table
Oncological outcome
The pathological tumor stage was similar in both groups (Table 4). There was no significant difference in the tumor-free distal margin between the groups, but the positive circumferential margin rate was slightly higher in the laparoscopic group although the difference was not statistically significant (Table 4).
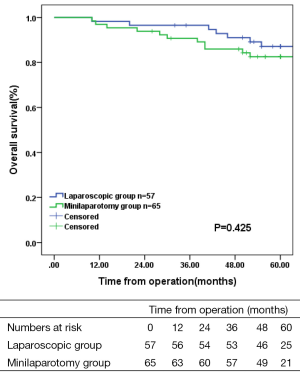
The mean follow up was 56.6 months (range, 10-84 months). There was no difference in local recurrence (5.3% vs. 1.5%, P=0.520) and distant recurrence (8.8% vs. 15.4%, P=0.267) between the two groups. Overall 5-year survival was 87.1% in the laparoscopic group and 82.5% in the minilaparotomy group (Figure 4; P=0.425). Disease-free survival in both groups is shown in Figure 5.

Full table

Discussion
This study comparing laparoscopic with open rectal cancer resection showed that the minilaparotomy approach was similarly safe and oncologically equivalent to laparoscopic approach, and performed with a shorter operative time and lower in-hospital costs than laparoscopic approach.
A previous study showed that the laparoscopic procedure for rectal cancer was associated with a more rapid postoperative recovery and better cosmetic results without compromising oncological outcomes as compared to open surgery (2). Our results also showed that the laparoscopic approach for rectal cancer was associated with an earlier resumption of normal diet and shorter hospital stay, and the time to first bowel movement was shorter in laparoscopic group, but not significant. Contrary to what has been reported previously, the present study failed to demonstrate lower pain scores for the laparoscopic group (15,16). An explanation could be the use of five ports and an about 5 cm abdominal incision for specimen retrieval in the laparoscopic group that might produce more wound pain. The more analgesic consumption might also limit postoperative recovery.
The postoperative complication rate was less in the laparoscopic group, but the difference did not reach significance. Anastomotic leak rate was 1.8% in the laparoscopic group and 3.1% in minilaparotomy group. This leak rate was similar to the results in other studies in the literature (1-13.5%) (4,16-19). Most of the long-term complications such as anastomotic stenosis, incisional hernia and urogenital dysfunctions were minor and the reoperation rate was low in both arms. This study therefore suggests that the minilaparotomy approach is as safe as the laparoscopic approach and does not lead to higher morbidity.
In the present study, the number of lymph nodes harvested was not different between the two groups. The distance between the tumor and distal resection margin was slightly less in the laparoscopic group and the rate of involved circumferential margin was higher, although these differences were not statistically significant. This finding is similar to the findings in the CLASICC-trial where the circumferential margin involvement rate was 12% in the laparoscopic group and 6% in the open group (P>0.05) (4,17). The CLASICC trial suggested that laparoscopic LAR could be associated with a slightly increased risk of local recurrence (4). However, recent studies suggested laparoscopic results showed equal distal margin length and the rate of margin positivity when compared to open surgery (20). A possible explanation may be that we did not have a longer learning curve and enough experience, and could not obtain enough distal length and locate the tumor to accomplish the anastomosis in many very low rectal cancer patients. Furthermore, in laparoscopic surgery, we used linear stapler which cannot bend at the distal shaft. It was very difficult for us to get longer distal margin in low rectal patients with narrow pelvis. The third reason may be that the tumors were slightly more distal and lower in the laparoscopic group compared with the minilaparotomy group.
Conversion to an open operation is an important indicator for laparoscopic success. The conversion rate was 8.8%, which was similar to the rates reported in the literature (6-15.5%) (16,21-23). Factors predictive of conversion are the size of tumor, bleeding, the experience of the surgeon, and the inability to localize small tumors (24). In this study, the major cause for conversion was an inadequate laparoscopic resection leading to an inadequate excision. Preoperative colonoscopic tattooing was a safe and effective method for tumor localization in laparoscopic colorectal surgery (25). Intraoperative colonoscopy was also a way of definitively localizing a lesion (26).
Port site recurrence has been reported after laparoscopic resection of colorectal cancer (0-1.4%) (24,27). In the present study, there was no port site recurrence. More importantly, there was no difference in overall and disease-free survival between minilaparotomy and laparoscopic group, and local and distant recurrence rates were similar in both groups. Similar results that supported the equivalence of oncologic outcomes have been reported in several single-institution comparative or randomized controlled studies (16,17,28). This study indicates that the minilaparotomy approach is oncologically feasible.
In this study, splenic flexure mobilization was conducted when necessary in the laparoscopic approach, but could not be performed in the minilaparotomy approach because of small incision. Some surgeons, especially those in Western countries, have suggested that wide splenic flexure mobilization was crucial to obtain adequate resection with tension-free anastomosis in rectal cancer surgery (29). However, we found that most patients need not splenic flexure mobilization to complete the anastomosis in the minilaparotomy approach, unless some patients with very short sigmoid colon and large quantities of mesentery fat. Some investigators from Asian countries have shown that Laparoscopic and open procedures without routine splenic flexure mobilization in the treatment of rectal cancer was feasible and did not seem to increase postoperative morbidity or oncologic risk (30,31).
The patients in minilaparotomy group were not overweight, because obesity was the risk factor preventing the success of the minilaparotomy approach in the resection of colorectal cancer (32), and almost all surgeons seem to agree that obesity reduced the technical feasibility of the minimally invasive laparoscopic and minilaparotomy approaches (3,10,11). Since the incidence of overweight or morbidly obese patients in Asia is probably lower than in Western countries (12,33), we feel that minilaparotomy is a suitable technique for many Asian patients with rectal cancer.
In conclusion, minilaparotomy approach is comparable to the laparoscopic approach in terms of postoperative complications and oncological outcomes, demonstrating the feasibility and the efficacy of the minilaparotomy approach. Laparoscopic approach has an advantage over minilaparotomy approach in allowing earlier recovery. However, this is at the expense of a longer operating time and higher direct costs. The minilaparotomy approach for resection of rectal cancer is an attractive alternative in highly selected patients. A criticism of the study is the fact that allocation of the patients was not random, which may present potential biases. A large-scale randomized trial for comparison of minilaparotomy and laparoscopic rectal cancer surgeries is needed. Careful patient selection is also crucial.
Acknowledgements
Disclosure: The authors have no conflicts of interest.
References
- Jayne DG, Thorpe HC, Copeland J, et al. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal Cancer. Br J Surg 2010;97:1638-45. [PubMed]
- Nelson H, Sargent D, Wieand HS, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [PubMed]
- Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005;6:477-84. [PubMed]
- Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718-26. [PubMed]
- Lacy AM, García-Valdecasas JC, Delgado S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 2002;359:2224-9. [PubMed]
- Avital S, Hermon H, Greenberg R, et al. Learning curve in laparoscopic colorectal surgery: our first 100 patients. Isr Med Assoc J 2006;8:683-6. [PubMed]
- Li JC, Leung KL, Ng SS, et al. Laparoscopic-assisted versus open resection of right-sided colonic cancer--a prospective randomized controlled trial. Int J Colorectal Dis 2012;27:95-102. [PubMed]
- Baik SH, Gincherman M, Mutch MG, et al. Laparoscopic vs open resection for patients with rectal cancer: comparison of perioperative outcomes and long-term survival. Dis Colon Rectum 2011;54:6-14. [PubMed]
- Hsu TC. Feasibility of colectomy with mini-incision. Am J Surg 2005;190:48-50. [PubMed]
- Ishida H, Nakada H, Yokoyama M, et al. Minilaparotomy approach for colonic cancer: initial experience of 54 cases. Surg Endosc 2005;19:316-20. [PubMed]
- Nakagoe T, Sawai T, Tsuji T, et al. Colectomy for colon cancer via a 7-cm minilaparotomy. Surg Today 2001;31:1113-5. [PubMed]
- Nakagoe T, Sawai T, Tsuji T, et al. Early outcome after minilaparotomy for the treatment of rectal cancer. Eur J Surg 2001;167:705-10. [PubMed]
- Fleshman JW, Fry RD, Birnbaum EH, et al. Laparoscopic-assisted and minilaparotomy approaches to colorectal diseases are similar in early outcome. Dis Colon Rectum 1996;39:15-22. [PubMed]
- Li S, Chi P, Lin H, et al. Long-term outcomes of laparoscopic surgery versus open resection for middle and lower rectal cancer: an NTCLES study. Surg Endosc 2011;25:3175-82. [PubMed]
- Leung KL, Kwok SP, Lam SC, et al. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet 2004;363:1187-92. [PubMed]
- Ng SS, Leung KL, Lee JF, et al. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol 2008;15:2418-25. [PubMed]
- Jayne DG, Guillou PJ, Thorpe H, et al. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol 2007;25:3061-8. [PubMed]
- Ströhlein MA, Grützner KU, Jauch KW, et al. Comparison of laparoscopic vs. open access surgery in patients with rectal cancer: a prospective analysis. Dis Colon Rectum 2008;51:385-91. [PubMed]
- Zhou ZG, Hu M, Li Y, et al. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc 2004;18:1211-5. [PubMed]
- Sara S, Poncet G, Voirin D, et al. Can adequate lymphadenectomy be obtained by laparoscopic resection in rectal cancer? Results of a case-control study in 200 patients. J Gastrointest Surg 2010;14:1244-7. [PubMed]
- Liang JT, Lai HS, Lee PH. Laparoscopic pelvic autonomic nerve-preserving surgery for patients with lower rectal cancer after chemoradiation therapy. Ann Surg Oncol 2007;14:1285-7. [PubMed]
- Poon JT, Law WL. Laparoscopic resection for rectal cancer: a review. Ann Surg Oncol 2009;16:3038-47. [PubMed]
- Laurent C, Leblanc F, Gineste C, et al. Laparoscopic approach in surgical treatment of rectal cancer. Br J Surg 2007;94:1555-61. [PubMed]
- Scheidbach H, Schneider C, Konradt J, et al. Laparoscopic abdominoperineal resection and anterior resection with curative intent for carcinoma of the rectum. Surg Endosc 2002;16:7-13. [PubMed]
- Park JW, Sohn DK, Hong CW, et al. The usefulness of preoperative colonoscopic tattooing using a saline test injection method with prepackaged sterile India ink for localization in laparoscopic colorectal surgery. Surg Endosc 2008;22:501-5. [PubMed]
- Cho YB, Lee WY, Yun HE, et al. Tumor localization for laparoscopic colorectal surgery. World J Surg 2007;31:1491-5. [PubMed]
- Morino M, Parini U, Giraudo G, et al. Laparoscopic total mesorectal excision: a consecutive series of 100 patients. Ann Surg 2003;237:335-42. [PubMed]
- Bretagnol F, Lelong B, Laurent C, et al. The oncological safety of laparoscopic total mesorectal excision with sphincter preservation for rectal carcinoma. Surg Endosc 2005;19:892-6. [PubMed]
- Tsang WW, Chung CC, Kwok SY, et al. Laparoscopic sphincter-preserving total mesorectal excision with colonic J-pouch Reconstruction: five-year results. Ann Surg 2006;243:353-8. [PubMed]
- Park JS, Kang SB, Kim DW, et al. Laparoscopic versus open resection without splenic flexure mobilization for the treatment of rectum and sigmoid cancer: a study from a single institution that selectively used splenic flexure mobilization. Surg Laparosc Endosc Percutan Tech 2009;19:62-8. [PubMed]
- Kim J, Choi DJ, Kim SH. Laparoscopic rectal resection without splenic flexure mobilization: a prospective study assessing anastomotic safety. Hepatogastroenterology 2009;56:1354-8. [PubMed]
- Nakagoe T, Matsuo T, Nakamura S, et al. Risk factors preventing success of a minilaparotomy approach in the resection of colorectal cancer. Dig Surg 2009;26:236-42. [PubMed]
- Cheng TO. Chinese body mass index is much lower as a risk factor for coronary artery disease. Circulation 2004;109:e184; author reply e184.