Early experience with cytoreduction and hyperthermic intraperitoneal chemotherapy at a newly developed center for peritoneal malignancy
Introduction
Peritoneal carcinomatosis (PC) was once considered a terminal condition with a poor prognosis and limited, if any, treatment options. The natural history is that of progressive abdominal distention due to the accumulation of ascites, abdominal pain, uncontrolled tumor growth, malignant bowel obstruction and ultimately death. Systemic chemotherapy has limited efficacy and the survival of patients with PC is approximately 6 months without treatment (1). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) has emerged as an accepted treatment modality for patients with intraperitoneal mesothelioma and PC from gastrointestinal and gynecologic malignancies (2-4). Consensus statements defining patient selection, pathology treated and technique of HIPEC delivery have been published in an attempt to standardize the management of these patients (5,6).
CRS/HIPEC is a complex procedure associated with potential significant morbidity, hospital length of stay and mortality (7). Gaining proficiency in cytoreductive procedures is difficult and optimal outcome is dependent on several variables including primary pathology, extent of disease, prior treatment, patient selection and surgeon experience, to name a few. There is an increasing interest in identifying a learning curve in the development of optimal proficiency for CRS/HIPEC procedures (8). A multi-institutional review of the Peritoneal Surface Oncology Group International (PSOGI) identified that 100 procedures per center were necessary to develop optimal expertise for the management of pseudomyxoma peritonei (9). Investigators from the University of Pittsburgh determined that 180 procedures were necessary to reduce incomplete cytoreduction and morbidity rates (10). Indeed, larger centers have been able to improve outcomes over time as a result of better patient selection, increased operative experience with more complete cytoreduction performed and fewer serious complications observed (11).
How to obtain similar operative and oncologic outcomes in a newly developed peritoneal surface malignancy program, however, remains a challenge. As the incidence of gastrointestinal cancers increases and with expanding indications for CRS/HIPEC, the need for new peritoneal surface malignancy centers that are able to achieve similar operative and oncologic outcomes consistent with larger, more established centers will be necessary. The University of Tennessee Health Science Center (UTHSC, Memphis, TN) Division of Surgical Oncology, in collaboration with the Methodist LeBonheur Healthcare system, St. Jude Children’s Research Hospital and West Cancer Center, developed a peritoneal surface malignancy program in 2011. During the first 5 years of our program, patient volume and experience have continued to grow. We report our early experience of the first 50 patients who underwent CRS/HIPEC and review our short-term perioperative outcomes. We hypothesize that a newly established peritoneal surface malignancy program can achieve early technical proficiency and similar operative outcome results consistent with more established centers.
Methods
A retrospective review was performed of a prospectively maintained database for patients who underwent CRS/HIPEC for management of peritoneal surface malignancies from December 2011 to November 2015. All patients were presented in multidisciplinary conference and were considered for CRS/HIPEC if he/she had a good performance status (Eastern Cooperative Oncology Group 0/1), no extra-peritoneal disease and were determined to be potential resection candidates based on preoperative imaging. Institutional review board approval was obtained at St Jude Children’s Research Hospital (XPD17-059) and the University of Tennessee Health Science Center (17-05205-XP).
The burden of disease, as defined by the peritoneal cancer index (PCI), was calculated at the completion of adhesiolysis. The abdomen is divided in to 13 regions with each region given a numeric score [0–3] with 0—no visible disease, 1—disease <0.5 cm, 2—disease <5 cm and 3—disease >5 cm and/or tumor confluence (12). PCI scores range from 0–39. The completeness of cytoreduction (CCR) score was determined at the completion of cytoreduction and prior to HIPEC (13). A CCR-0 score indicates no visible/macroscopic disease; CCR-1 score indicates tumor nodules <2.5 mm in diameter; CCR-2 score indicates nodules >2.5 mm but <2.5 cm in diameter; CCR-3 score indicates nodules >2.5 cm in diameter. Cytoreduction was performed as previously described (14,15). HIPEC was performed via a closed technique after the removal of all possible disease. Anastomoses, stoma creation and drain placement were performed at the completion of HIPEC. Patients were monitored in the intensive care unit postoperatively and transferred to the ward when appropriate. Postoperative morbidity was graded using the Clavien-Dindo schema (16). Follow up was performed at 2 weeks, 6 weeks, 3 months, 6 months, then at 6 month intervals thereafter. Follow up examination included physical examination, routine blood work including tumor markers (CEA, CA 125 and CA 19-9) and cross-sectional imaging (computed tomography-CT and/or magnetic resonance imaging-MRI).
Long-term follow up and outcome data were available for all patients who underwent CRS/HIPEC. Overall survival (OS) was defined as the duration of time from CRS/HIPEC to death or date of last follow up (months). Frequencies were characterized using the median (range) or mean (standard deviation), where appropriate. For comparison, the first 25 HIPEC procedures where compared to the second 25 HIPEC procedures. Categorical variables were evaluated using chi square method while continuous variables were analyzed with independent t-test. Kaplan-Meier method was used to calculate OS for the most common pathologies treated. A P value of <0.05 was considered significant. Statistical analysis was performed using SPSS software, version 24 (IBM Corporation, Armonk, NY).
Results
A total of 73 patients underwent evaluation for PC during the study period of which 50 underwent successful CRS/HIPEC and 23 patients (31%) were deemed unresectable secondary to advanced disease/other comorbidities. We further investigated the outcome for the first 50 patients who underwent CRS/HIPEC. The median age was 53.5 years (range, 11–73 years) and a majority (64%) were Caucasian. Thirty-six patients (72%) had undergone prior laparotomy while 76% had received prior systemic chemotherapy. Three patients had undergone prior CRS/HIPEC. The most common pathologies treated included: appendix (40%), ovary (20%), colon (14%), desmoplastic small round cell tumor (DSRCT, 14%) or other (12%). The median time to CRS/HIPEC was 6 months from the date of diagnosis (Table 1).

Full table
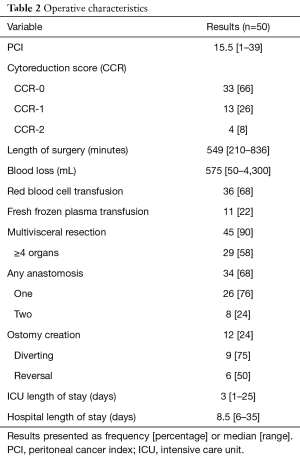
The median PCI was 15.5 (range, 1–39) and a CCR 0/1 resection was performed in 92%. Preoperative cystoscopy and bilateral ureteral catheter placement was performed in a majority (90%) at the time of CRS/HIPEC. The CRS/HIPEC procedure lasted a median of 549 minutes (range, 210–836 minutes) with a median of 575 mL of blood loss (range, 50–4,300 mL). Forty-five patients (90%) underwent multivisceral resection with 58% undergoing resection of 4 or more organs. Twelve patients (24%) required stoma placement at the time of CRS/HIPEC of which half subsequently underwent reversal. Postoperatively, all patients were monitored in the ICU (median 3 days; range, 1–25 days) with a median hospital length of stay of 8.5 days (range, 6–35 days, Table 2).
Full table
Twenty-two patients (44%) had some form of complication of which 16% were classified as major (Clavien-Dindo 3–5). Patients who suffered a major complication were more likely to have a longer ICU length of stay (8.1 vs. 2.7 days, P<0.001), while age, PCI score, length of surgery, blood loss, multivisceral resection or number of anastomoses had no significant impact. Four patients (8%) required transfer to a rehabilitation or skilled nursing facility and 13 patients (26%) were re-admitted within 30 days of discharge after CRS/HIPEC. Two patients died within the 30-day postoperative period, one from a pulmonary embolus on day 6 and the other from multisystem organ failure secondary to an anastomotic leak on day 9. Adjuvant radiation was administered to 12 patients (all with DSRCT pathology) while 66% received adjuvant chemotherapy.
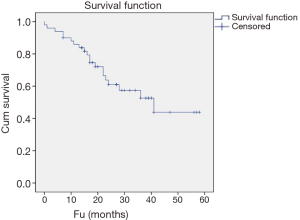
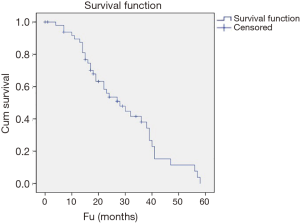
With a median follow up of 22 months (range, 0–58 months), 30% were alive with no evidence of disease and 30% alive with disease. Twenty patients died either from their disease (34%) or from another cause (6%). Thirty-six patients (72%) developed recurrence of disease with a median of 12 months (range, 0–43 months) to development of recurrence. Ten patients (20%) developed distant disease with a median time to development of distant disease of 6 months (range, 0–22 months; Table 3). The 1- and 3-year OS was 86% and 53% while the 1- and 3-year RFS was 81% and 45%, respectively (Figures 1,2).
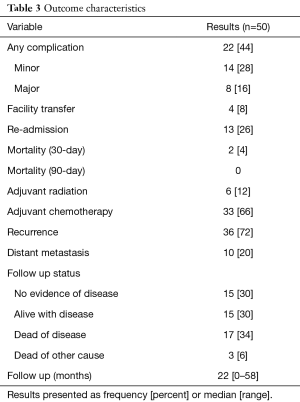
Full table
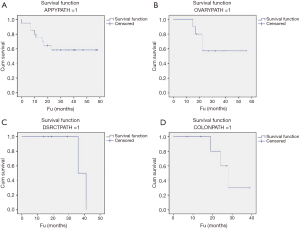
The mean OS based on primary pathology was as follows: appendix pathology (38.8 months; 95% CI, 28.4–49.1 months), ovary pathology (40.2 months; 95% CI, 28.4–52.0 months), DSRCT (38.5 months; 95% CI, 33.6–43.4 months) and colon pathology (28.7 months; 95% CI, 21.7–35.7 months; Figure 3A,B,C,D). We examined the treatment or outcome for the first 25 patients and compared them to the second set of 25 patients who underwent CRS/HIPEC. There were no major differences in treatment or outcome for either group of patients during the study period (1st 25 patients versus 2nd 25 patients). The PCI score was higher and the length of the CRS/HIPEC procedure was longer for the first group of 25 patients, both of which were significant. The length of follow up was shorter for the 2nd 25 patients who underwent CRS/HIPEC, although the follow up data is not as mature as for the more recent cohort (Table 4).
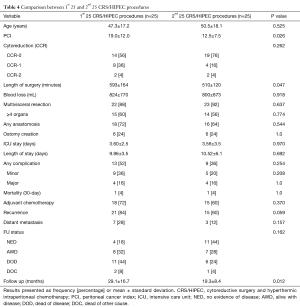
Full table
Discussion
As a new center for peritoneal surface malignancies with less than 5 years of experience, our goal at the creation of the program was to emulate the operative and oncologic results of more established centers. These centers began similar to ours and as they have continued to grow have developed into national referral centers for PC (10,11). As such, their collective experience has set the standard of care for operative expectations and oncologic outcomes for newer centers who begin to perform CRS/HIPEC.
CRS/HIPEC procedures were performed at our institution by either one of two fellowship trained surgical oncologists who both had exposure to peritonectomy procedures during general surgery residency and surgical oncology fellowship. Procedures were often double scrubbed during the more difficult portions of the procedure, such as clearing of the pelvic disease and/or stripping of the diaphragm, as these are often the most difficult and time intensive portions of the procedure (15,17). We determined that we were able to maintain operative cadence, reduce blood loss and shorten the more lengthy portions of the procedure using a two attending surgeon technique. While having two-surgeons with peritonectomy experience during training was an early advantage, gaining the clinical judgement and technical expertise needed to achieve excellent outcomes requires additional exposure and time. Others have determined that obtaining technical proficiency is an acquired skillset and involves a lengthy learning process, dependent on several variables, most notably case volume (9). While our case volume has steadily grown each year, Polanco and colleagues noted that 180 cases were necessary to reduce the rate of incomplete cytoreduction while 90 cases were required to have an effect on oncologic outcomes (10). Voron and colleagues reported on 204 patients who underwent CRS/HIPEC identifying that 140 cases were needed to achieve technical proficiency and reduce major morbidity (18).
Our center is in its infancy compared to these larger experiences, however, our early perioperative outcomes do yield some positive insights, specifically with respect to cytoreduction and short-term complications. The CCR score is useful as an indirect surrogate of technical proficiency and an important predictor of outcome in patients who are successfully able to undergo CRS/HIPEC (19). We were able to achieve optimal cytoreduction (CCR 0/1) in 92% of patients. This compares favorably with other new as well as established centers able to achieve CCR 0/1 rates ranging from 70–87% (9,10,18,20,21).
Postoperative morbidity and mortality are also crucial determinants of outcome and useful variables in defining proficiency of CRS/HIPEC procedures. These procedures are inherently of long duration and associated with significant morbidity and mortality, ranging from 24–34% and 2–4%, respectively (22-24). In review of our early experience, 28% of patients suffered a minor (Clavien-Dindo 1–2) complication with 16% suffering a major (Clavien-Dindo 3–5) complication and two patients died (4%). These results are congruent with other reported series. In a multicenter study of 1,290 patients with nonovarian pathology, Glehen and colleagues reported a major postoperative complication (grade 3 and 4) occurring in 403 patients (33.6%) with a perioperative mortality occurring in 52 (4.1%) (25). Increasing patient age and the extent of carcinomatosis (PCI score) were independent predictors of major morbidity and mortality. Our results also compare similarly to other new centers who have been able to achieve low morbidity and low perioperative mortality (20,21). Several independent predictors of morbidity and mortality have been identified including age, operative time, extent of disease, completeness of cytoreduction, number of anastomoses, HIPEC center, tumor location and grade, as well as others (10,23,26,27). We were unable to identify any factors associated with major morbidity or mortality, presumably because of the small number of patients, but did observe a higher ICU length of stay in those with a major complication. Recently, a novel tool to predict major morbidity and identify low-risk patients who are likely to benefit from CRS/HIPEC has been described and may be useful during preoperative counseling of patients with PC (28).
As a new CRS/HIPEC center, we have also been fortunate to collaborate with St Jude Children’s Research Hospital in the management of pediatric and young adult patients with desmoplastic small round cell tumor (DSRCT). DSRCT is a rare abdominal sarcoma with peritoneal spread and frequently affects adolescent males. DSRCT is an aggressive histology with a dismal historical prognosis. Conventional treatment consisting of systemic chemotherapy and surgical debulking now includes CRS/HIPEC, which has demonstrated a disease-free survival and OS benefit in appropriately selected patients (29-31). One-year survival rates of almost 90% have been achieved with CRS/HIPEC and those who are able to undergo complete cytoreduction have the best prognosis (32). Seven of the first 50 CRS/HIPEC procedures performed in our early experience were for DSRCT. Because the risk of recurrence is high, many go onto additional therapy including adjuvant systemic chemotherapy, whole abdomen radiation, and autologous stem cell transplantation (33,34). At last follow up, two patients have passed away of disease (39 and 41 months) while 5 remain alive, 3 with disease (FU of 17, 14 & 14 months) and 2 free of disease (FU of 29 & 19 months; Figure 3C). The long-term impact of CRS/HIPEC for DSRCT remains to be determined but does appear to offer encouraging results.
One of the many challenges we have had to address as a new center has been the evaluation and successful completion of CRS/HIPEC procedures. As an example, 73 patients underwent evaluation for PC of which almost one-third were deemed unresectable at the time of exploration. A majority of patients had advanced disease precluding CCR 0/1 resection, often due to extensive small bowel serosal involvement or a fused mesentery. These patients, therefore, were required to recover from a nontherapeutic laparotomy before resuming palliative chemotherapy. The decision to abort cytoreduction and not proceed with HIPEC was often made in consultation with a second surgeon. While some have noted that a nontherapeutic laparotomy in the setting of attempted CRS does not negatively affect outcome and/or resumption of chemotherapy, we feel this represents an area for improvement (35). Several risk factors for incomplete cytoreduction have been described including a high burden of disease, primary tumor origin, signet ring histology and tumor location (25,36,37). As we examined our experience, patients who had more disease on pre-operative imaging and colon pathology were more likely to undergo aborted procedures. We determined that diagnostic laparoscopy was a potential diagnostic modality which could help us reduce the percentage of aborted CRS attempts. Conventional cross-sectional imaging may under represent the exact burden of disease identified at the time of exploration and for this reason several have advocated the use of laparoscopy in the evaluation of patients with PC (38). Diagnostic laparoscopy is a low morbidity procedure that, even in the setting of prior operation, often allows complete assessment of the abdominal cavity (39). While we did not routinely perform diagnostic laparoscopy at the onset of our experience, we have since begun to incorporate laparoscopy in the management of patients with PC. Nineteen laparoscopy procedures have since been performed prior to CRS/HIPEC for colorectal and high-grade appendiceal pathology with 11 patients who have gone onto CRS/HIPEC and the remaining undergoing palliative chemotherapy.
Our preoperative evaluation and postoperative management of patients who have undergone CRS/HIPEC has also continued to evolve over time. When reviewing our early outcomes, we observed that 1 in 4 patients required re-admission during the perioperative period, often for anorexia, failure to thrive and generalized deconditioning. In an effort to be more proactive, we subsequently developed a preoperative nutritional and physical therapy assessment program. Patients undergo consultation preoperatively with a clinical nutritionist and a licensed physical therapist to assess for underlying protein-energy malnutrition, address specific dietary intake concerns and improve preoperative performance status and exercise tolerance. While patients seem to appreciate a comprehensive approach, we hope these programs will yield beneficial results in the future, improving recovery and reducing the need for readmission and rehabilitation. We have also hired a HIPEC coordinator (DD & DF) to assist with the management of these complex patients, before, during and after surgery. The coordinator has proven instrumental in facilitating patient communication, scheduling of preoperative studies, ensuring adherence to protocols, postoperative examination and long-term surveillance. The HIPEC Coordinator also runs the weekly multidisciplinary patient conference which consists of nursing, nutrition, physical therapy, pharmacy, case management and anesthesia personnel as well as our monthly peritoneal surface malignancy support group. Lastly, we have also initiated a goal directed fluid therapy protocol over the last two years with the Department of Anesthesia to improve perioperative recovery as others have demonstrated a reduced risk of major complications and hospital length of stay when compared to standard fluid therapy (40).
There are several important limitations to this study. The retrospective nature and short follow up limit conclusions regarding outcomes after CRS/HIPEC as the treatment is not randomized. Similarly, surgeon experience and operative judgement certainly affected outcome and treatment decisions. When to proceed with cytoreduction or when to abort has continued to evolve over time and based upon our growing experience. The heterogeneous patient population, consisting of pediatric patients up to more advanced ages, includes a variety of pathologies with differing grades, biologic behavior, methods and duration of treatment. While all patients are presented in a multidisciplinary tumor board manner, the lack of standardized treatment amongst pathologies adds variability and confounds results.
Conclusions
Short-term outcomes observed after CRS/HIPEC in a newly developed center for PC are consistent with published higher volume center experiences. Appropriate patient selection, selective use of laparoscopy, institution of goal directed fluid therapy protocols with preoperative nutritional and physical therapy assessments will hopefully yield improved outcomes as experience develops. Establishing a HIPEC Coordinator has proven instrumental in facilitating patient coordination, scheduling of preoperative studies and adherence to protocols.
Acknowledgements
The authors wish to acknowledge Donna Freeman, RN who provided support and encouragement to the patients undergoing treatment in this newly established peritoneal surface malignancy program.
Footnote
Conflicts of Interest: This work was presented in part at the 69th Southwestern Surgical Congress annual meeting, April 2-5, 2017, Maui, HI.
Ethical Statement: Institutional review board approval was obtained at St Jude Children’s Research Hospital (XPD17-059) and the University of Tennessee Health Science Center (17-05205-XP). Informed consent was not obtained as this work represents a retrospective review.
References
- Glehen O, Osinsky D, Beaujard AC, et al. Natural history of peritoneal carcinomatosis from nongynecologic malignancies. Surg Oncol Clin N Am 2003;12:729-39. xiii. [Crossref] [PubMed]
- Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol 2007;14:128-133. [Crossref] [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [Crossref] [PubMed]
- Elias D, Gilly F, Quenet F, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol 2010;36:456-62. [Crossref] [PubMed]
- Esquivel J, Elias D, Baratti D, et al. Consensus statement on the loco regional treatment of colorectal cancer with peritoneal dissemination. J Surg Oncol 2008;98:263-7. [Crossref] [PubMed]
- Turaga K, Levine E, Barone R, et al. Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol 2014;21:1501-5. [Crossref] [PubMed]
- Kusamura S, Younan R, Baratti D, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer 2006;106:1144-53. [Crossref] [PubMed]
- Moradi BN 3rd, Esquivel J. Learning curve in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol 2009;100:293-6. [Crossref] [PubMed]
- Kusamura S, Moran BJ, Sugarbaker PH, et al. Multicentre study of the learning curve and surgical performance of cytoreductive surgery with intraperitoneal chemotherapy for pseudomyxoma peritonei. Br J Surg 2014;101:1758-65. [Crossref] [PubMed]
- Polanco PM, Ding Y, Knox JM, et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann Surg Oncol 2015;22:1673-9. [Crossref] [PubMed]
- Levine EA, Stewart JH, Shen P, et al. Intraperitoneal chemotherapy for peritoneal surface malignancy: experience with 1,000 patients. J Am Coll Surg 2014;218:573-85. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [Crossref] [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [Crossref] [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Surg Oncol Clin N Am 2003;12:703-27. xiii. [Crossref] [PubMed]
- Bao P, Bartlett D. Surgical techniques in visceral resection and peritonectomy procedures. Cancer J 2009;15:204-11. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Ahmed S, Levine EA, Randle RW, et al. Significance of diaphragmatic resections and thoracic chemoperfusion on outcomes of peritoneal surface disease treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 2014;21:4226-31. [Crossref] [PubMed]
- Voron T, Eveno C, Jouvin I, et al. Cytoreductive surgery with a hyperthermic intraperitoneal chemotherapy program: Safe after 40 cases, but only controlled after 140 cases. Eur J Surg Oncol 2015;41:1671-7. [Crossref] [PubMed]
- Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol 2005;2:3. [Crossref] [PubMed]
- Chang KH, Kazanowski M, Staunton O, et al. Mentored experience of establishing a national peritoneal malignancy programme - Experience of first 50 operative cases. Eur J Surg Oncol 2017;43:395-400. [Crossref] [PubMed]
- Konstantinidis IT, Young C, Tsikitis VL, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion: The University of Arizona early experience. World J Gastrointest Surg 2012;4:135-40. [Crossref] [PubMed]
- Sugarbaker PH, Alderman R, Edwards G, et al. Prospective morbidity and mortality assessment of cytoreductive surgery plus perioperative intraperitoneal chemotherapy to treat peritoneal dissemination of appendiceal mucinous malignancy. Ann Surg Oncol 2006;13:635-44. [Crossref] [PubMed]
- Bartlett EK, Meise C, Roses RE, et al. Morbidity and mortality of cytoreduction with intraperitoneal chemotherapy: outcomes from the ACS NSQIP database. Ann Surg Oncol 2014;21:1494-500. [Crossref] [PubMed]
- Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63-8. [Crossref] [PubMed]
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 2010;116:5608-18. [Crossref] [PubMed]
- Chua TC, Moran BJ, Sugarbaker PH, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 2012;30:2449-56. [Crossref] [PubMed]
- Desantis M, Bernard JL, Casanova V, et al. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC). Langenbecks Arch Surg 2015;400:37-48. [Crossref] [PubMed]
- Baumgartner JM, Kwong TG, Ma GL, et al. A Novel Tool for Predicting Major Complications After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy. Ann Surg Oncol 2016;23:1609-17. [Crossref] [PubMed]
- Kushner BH, LaQuaglia MP, Wollner N, et al. Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol 1996;14:1526-31. [Crossref] [PubMed]
- Lal DR, Su WT, Wolden SL, et al. Results of multimodal treatment for desmoplastic small round cell tumors. J Pediatr Surg 2005;40:251-5. [Crossref] [PubMed]
- Hayes-Jordan A, Green H, Fitzgerald N, et al. Novel treatment for desmoplastic small round cell tumor: hyperthermic intraperitoneal perfusion. J Pediatr Surg 2010;45:1000-6. [Crossref] [PubMed]
- Hayes-Jordan A, Green H, Lin H, et al. Cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for children, adolescents, and young adults: the first 50 cases. Ann Surg Oncol 2015;22:1726-32. [Crossref] [PubMed]
- Forlenza CJ, Kushner BH, Kernan N, et al. Myeloablative chemotherapy with autologous stem cell transplant for desmoplastic small round cell tumor. Sarcoma 2015;2015:269197. [PubMed]
- Pinnix CC, Fontanilla HP, Hayes-Jordan A, et al. Whole abdominopelvic intensity-modulated radiation therapy for desmoplastic small round cell tumor after surgery. Int J Radiat Oncol Biol Phys 2012;83:317-26. [Crossref] [PubMed]
- Rodt AP, Svarrer RO, Iversen LH. Clinical course for patients with peritoneal carcinomatosis excluded from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2013;11:232. [Crossref] [PubMed]
- Glehen O, Mohamed F, Sugarbaker PH. Incomplete cytoreduction in 174 patients with peritoneal carcinomatosis from appendiceal malignancy. Ann Surg 2004;240:278-85. [Crossref] [PubMed]
- Winer J, Zenati M, Ramalingam L, et al. Impact of aggressive histology and location of primary tumor on the efficacy of surgical therapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2014;21:1456-62. [Crossref] [PubMed]
- Jayakrishnan TT, Zacharias AJ, Sharma A, et al. Role of laparoscopy in patients with peritoneal metastases considered for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC). World J Surg Oncol 2014;12:270. [Crossref] [PubMed]
- Marmor RA, Kelly KJ, Lowy AM, Baumgartner JM. Laparoscopy is Safe and Accurate to Evaluate Peritoneal Surface Metastasis Prior to Cytoreductive Surgery. Ann Surg Oncol 2016;23:1461-7. [Crossref] [PubMed]
- Colantonio L, Claroni C, Fabrizi L, et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg 2015;19:722-9. [Crossref] [PubMed]