Preoperative survival calculator for resectable hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the third leading cause of cancer related death worldwide (1,2). For patients with early stage disease, liver resection provides a potential for a cure but the overall prognosis is poor. Overall survival (OS) of HCC is affected not only by the aggressiveness of the type of tumor, but also the patient’s underlying liver disease, as up to 90% of HCC patients have been reported to have cirrhosis (3). Several clinical models of liver disease such as the Child-Pugh classification and Model for End-Stage Liver Disease (MELD) attempt to estimate the patients’ overall liver health and are used as part of the multidisciplinary and surgical evaluation of HCC patients (4,5). Due to the role of underlying liver dysfunction and the varying tumor biology, clinical staging systems such as tumor, node, and metastasis (TNM) and Cancer of the Liver Italian Program (CLIP) are less reliable as preoperative assessments for surgical resection.
Methods to predict morbidity and OS during the preoperative assessments can facilitate the decision making process. However, the development of propensity scores for estimating survival has been primarily from single or cooperative Asian and European institutions that combine both postsurgical factors and preoperative factors (6,7). There exists a paucity of literature for predicting oncologic outcomes for HCC patients based on preoperative factors from a US population or from a large nationwide database. Prediction of preoperative OS could potentially impact multidisciplinary decisions regarding further treatment recommendations for these complex patients. To this end, the purpose of this study was to develop a novel preoperative calculator that accurately predicted OS.
Methods
Patients
The American College of Surgeons National Cancer Data Base (NCDB), 1998–2012, was utilized to identify patients with HCC who had undergone surgical resection. As this study utilized a de-identified national database, it was deemed exempt from IRB review. The NCDB captures 70% of cancer related surgery in the United States. Although data existed for patients from 1998–2013 at the time of the study, survival data was only validated until 2012. Patients with HCC were identified using International Statistical Classification of Diseases (ICD O-3) codes 8170, 8171, 8173, 8174, 8175 and 8180.
Inclusion criteria consisted of patients who had TNM clinical stages 1–3, invasive behavior on pathology, and known vital status. Surgery of the primary site included wedge resection or segmental resection (NCDB site codes 20–25), right or left lobectomy (NCDB site codes 30, 36–37) and right or left extended lobectomy (NCDB site codes 50, 51–52). Patients who underwent segmental resection and local tumor ablation (NCDB site code 26), lobectomy and local tumor destruction (NCDB site code 38) and extended lobectomy and local tumor destruction (NCDB site codes 59) were excluded from analysis as the primary purpose was to develop a calculator for surgical resection only. It is difficult to compare patients who underwent ablation as this carries a higher risk for local recurrence and the indication for surgical ablation vs resection may also be due to underlying liver disease that is not available in dataset.
Local recurrence data is also not available from NCDB for HCC. To ensure exclusion of metastatic patients from analysis, patients with clinical or pathologic stage M1 disease were also excluded. Additionally, patients who had undergone previous treatment were excluded from the analysis.
Statistical analysis
In developing the OS calculator, the patients were randomly divided into building (nb) and validation (nv) cohorts using a 80/20 allocation using previously described methods (8,9). Descriptive statistics were reported using the mean, median and standard deviation for continuous variables; and using frequencies and relative frequencies for categorical variables. Cohorts were compared using Mann-Whitney U and Pearson’s chi-square tests for continuous and categorical variables respectively. Kaplan-Meier (KM) methods were used to summarize OS, from which estimates of median survival rates were obtained with 95% confidence intervals (CI).
The following analyses were conducted using the nb cohort. Univariate associations between OS and patient variables were examined using a Cox regression model. The models were fit using Firth’s penalized function and hazard ratios (HRs), with 95% CI, were obtained from model estimates. A prediction model was then developed using a multivariable Cox regression model, where the model form was developed in two steps. First, the main effects were chosen using a bootstrap backwards selection (BBS) method (alpha exit =0.1) with selected candidate variables as listed in the model. Second, all two-way interactions between the selected main effects were considered, and significant interactions were selected using the BBS method.
The final model included the selected main effects and two-way interactions; and model estimates were obtained using a multivariable Cox regression model fit using Firth’s method. The estimated baseline 1- and 3-year OS rates were obtained from the model using the BASELINE function available in SAS v9.4 software’s PHREG procedure, and corresponds to the estimated 1- and 3-year OS rate for individuals with reference-level covariate values. The final model’s parameter estimates and the corresponding baseline 1- and 3-year OS estimates were then used to generate 1- and 3-year OS prediction models. The models were recalibrated using standard bootstrap cross-validation methods. Model performance was assessed using time-dependent receiver operating characteristic (ROC) curves, the corresponding area under the ROC curve (AUC), and calibration plots (10).
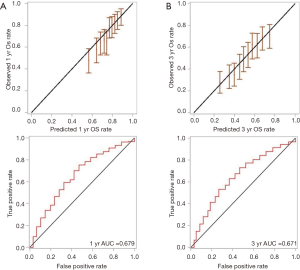
The 1- and 3-year OS prediction models were then applied to the nv cohort, with model performance assessed using ROC curves with corresponding AUC and calibration plots. The calibration plots for 1- and 3-year OS showed the predicted survival rates (grouped by deciles) on the horizontal axis versus the observed survival rates (with corresponding 95% CI) on the vertical axis. These calibration plots identify when the prediction models over, under or accurately predicted the OS rates. All analyses were conducted using SAS v9.4 (Cary, NC, USA), at a significance level of 0.05.
Results
Of 6,297 patients with invasive HCC, 5,455 patients met inclusion criteria and 4,364 (80%) patients were used in the nb cohort and 1,091 (20%) patients were used in the nv cohort. The majority of the patients had HCC “not otherwise specified” on histology, and the median size of the resected tumors was 6.0 cm (range, 0.5–9.9 cm). Comparison of and nb and nv cohorts based on these variables showed no statistical differences (Table 1). In the study population, 32.8% underwent wedge resection, 10.0% underwent partial hepatectomy, 49.9% underwent formal lobectomy, and 7.3% underwent extended lobectomy.
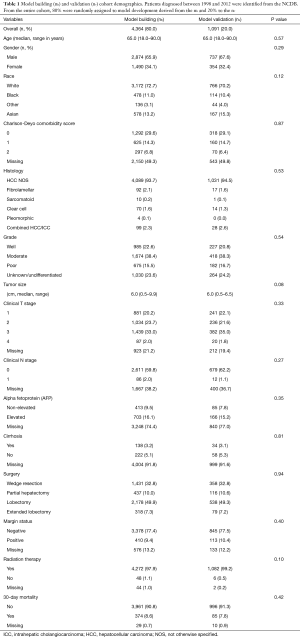
Full table
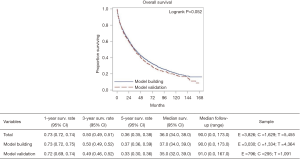
The median OS for the overall cohort was 36 months (95% CI, 34–38 months), and the median follow up was 90 months (95% CI, 0–173 months). There was no difference between the nb and nv cohorts with respect to median follow-up or median OS (Figure 1).
Several factors associated with OS were analyzed to build the calculator. Factors that were found to be significant on univariate analysis included age, gender, race, Charlson-Deyo score, tumor histology, tumor grade, tumor size, clinical T and N stage, AFP (alpha fetoprotein), presence of cirrhosis, and the degree of surgical resection (Table 2). Chemotherapy and radiation therapy were not found to be significant on univariate analysis, which is not unexpected since adjuvant therapy has not been shown to improve survival and thus not recommended by National Comprehensive Cancer Network (NCCN) guidelines.
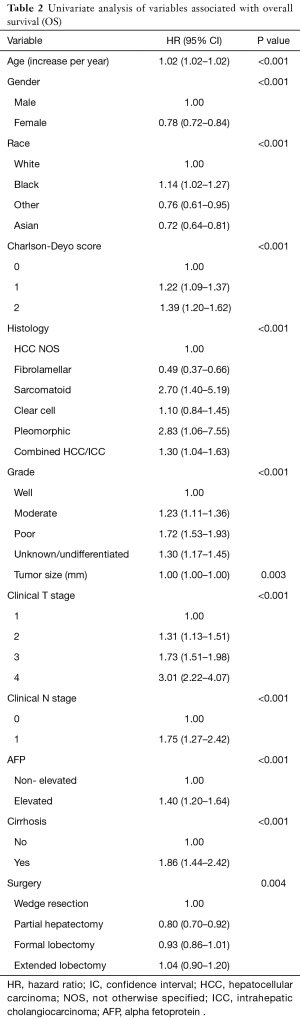
Full table
Although several preoperative factors including AFP, Charlson-Deyo score and presence of biopsy proven cirrhosis were found to be significant on univariate analysis, they were not included in the model. This is because more than 30% of the values were missing and thus would risk being misrepresented in the calculator. Therefore, using the BBS method, only the variables of age, gender, race, tumor histology, tumor grade, tumor size, clinical stage and type of surgery were included in the model.
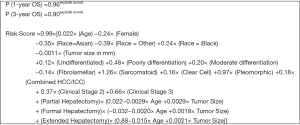
The equation derived from the OS prediction model is depicted in Figure 2. In assessing the accuracy of our model, the area under the ROC curve (AUC) for 1- and 3-year OS were 0.658 and 0.672 respectively, which suggests moderate performance of the model. There was minimal difference between the ROC and the AUC of the nb cohort compared to the nv cohort, with the latter AUC values for 1- and 3-year OS being 0.679 and 0.671, respectively (Figure 3). This suggested that the model performed reasonably well.
Discussion
Unlike other resectable tumors, the decision to proceed with resection for HCC is more complex. This is partly due to the variety of treatments available including radioembolization, transarterial chemoembolization (TACE), ablation, chemotherapy, surgical resection, or a combination of the therapies. Additionally, the preoperative and postoperative liver function complicates the assessment. The decision on the type of treatment is usually made by multidisciplinary groups. However, there is limited prospective data to compare these treatments to make strong evidence-based decisions at this time. Although both retrospective and prospective studies have shown that for early stage HCC (tumors <5 cm), surgical resection results in improved OS and lower recurrence rate than ablation, there are no prediction calculators that compare these treatments (11-15). For large Barcelona Clinic Liver Cancer (BCLC) stage A tumors, surgical resection has been reported to show improved OS compared to TACE in specific patients. However, the participants in the study were not prospectively matched (16). Some retrospective studies on highly selected patients suggest that for larger tumors (>7 cm), survival is equivalent for resection versus embolization-ablation. However, it is difficult to compare these methods based on known preoperative factors (17,18).
A calculator that estimates OS with known preoperative factors may allow for comparisons of different treatments and assist in the clinical decision making process. It also provides the patients an estimate of their outcome when considering surgical intervention. As an example, if the calculator predicts a low likelihood of 1- and 3-year OS after surgery, then less invasive treatments may be more strongly considered. This may help guide therapy for complex patients. As most retrospective studies show that patients with more advanced tumors are being considered for or undergoing surgical resection, surgery is often only recommended for those with smaller tumors and good liver performance (19,20). For patients with multifocal disease or extensive disease that requires a more complex operation, the benefit of surgery on OS is less well-established. Nonetheless, these resections are still performed and published especially within Asian centers (21). Therefore, preoperative survival estimates may have a substantial impact on decision making especially in these complex patients. Additionally, preoperative survival estimates can also be used as a gauge for transplantation (if the patient is a potential candidate) or interventional treatment.
Based on the current literature, the NCDB has not been used to create a preoperative OS calculator for HCC patients. The NCDB allows for a large database of surgically resected patients that captures 70% of cases in the United States. HCC calculators that have been developed were not based on US population databases. The NCDB has been effectively used to power a post-operative calculator for stage 2 and 3 colon cancer (9). Similar to this colon calculator, our HCC calculator takes into account several variables related to survival, like the degree of surgical resection, patient age, race, size of lesion, and the subtype of HCC if known. Input of this data into the calculator can provide an estimate of survival during the preoperative evaluation (22-24). Tumor size and tumor volume has also been suggested as factors that contribute to OS (25). It has been suggested that for patients with early tumors and unfavorable histologic features, anatomic resection can lead to lower rates of early recurrence and improved OS. Interestingly, for patients with well/moderately differentiated tumors, non-anatomic resection can have a similar benefit, decreasing local recurrence and improving OS (15,26-28). Our novel calculator allows the caregiver to compare the estimated OS depending on the extent of resection as part of preoperative planning.
There are several calculators that aim to estimate post-operative survival in HCC patients but are often used to compare other treatment modalities such as ablation or chemoembolization. It has been suggested that some perioperative factors may impact OS and are therefore maybe pertinent to survival calculators. Length of stay and postoperative complications are known to affect long term survival (29-31). However, neither of these items is known preoperatively so they were not pertinent to the formulation of our calculator. Additionally, prognostic biomarkers like those in the 5 gene signature were found to be predictive of recurrence and survival in French patients following resection (32). Gene signature data on HCC patients was not available for our model.
One Asian retrospective series identified a scoring system that incorporated preoperative values of prealbumin, alkaline phosphatase, AFP, tumor size >8 cm, platelet count and gamma-glutamyl transferase (GGT) revealing a 1-year morality rate of 62% in patients with scores ≥5 vs. a rate of 5% in patients with scores <5 (33). The patients in the former group also had a higher risk of microvascular invasion, poor tumor differentiation and cirrhosis. This scoring system, however, did not take into account the degree of resection. There are several studies that report an association of inflammatory markers with OS in resection patients including the Glasgow Prognostic Score and inflammation-based score (6,7). These features, however, were also not available for consideration in our model.
There are several limitations to our study. Although several preoperative factors including AFP, Charlson-Deyo score and presence of biopsy proven cirrhosis were found to be significant on univariate analysis, they were not included in the mode as the values were missing for most of the patients. Preoperative AFP is known to be associated with both disease free survival and OS (34). Although it is useful to know preoperative liver function status, the practice of liver biopsy to document cirrhosis is not uniform unless the patient is being considered for transplantation. These variables have been added with more recent data collections of the NCDB and may play a significant role in a future calculator.
With HCC, the causes of recurrence and death are multifactorial. They are related to both the tumor biology and intrinsic liver function. The power of our calculator is limited by the availability of information related to these factors. The NCDB only provides select elements of liver function as mentioned above and does not contribute related information such as platelet count, INR, Child-Pugh score, and others. The NCDB is also limited on data elements to accurately assess recurrence for HCC, a factor that would further benefit our calculator if known. To obtain an estimate of the OS during the preoperative setting, we purposefully excluded postoperative variables including the length of stay, postoperative complications, or recurrence. Exclusion of these variables may diminish the calculator’s accuracy of estimating the OS in the post-operative setting. However, this was not the intended purpose of our calculator.
Despite these limitations, using this calculator we aim to prospectively validate the results with patients from our institutional dataset. Furthermore, as NCDB collects more site specific factors, incorporating additional variables associated with tumor biology and liver function, our calculator’s ability to predict OS may be further enhanced. In conclusion, the decision to proceed with surgical resection as opposed to interventional treatment, chemotherapy, or transplantation involves consideration of the likelihood of cure, benefit of survival, technical feasibility, as well as the ability of the patient’s liver to tolerate resection. Our preoperative calculator incorporates known factors to assess OS and performs reasonably well in order to contribute to clinical decision-making.
Acknowledgements
We acknowledge and thank the American College of Surgeons Committee on Cancer for providing access to the Participant User File from the National Cancer Data Base.
Funding: This work was supported by NCI grant P30CA016056 involving the use of RPCI’s Biostatistics Shared Resource.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As this study utilized a de-identified national database, it was deemed exempt from IRB review.
References
- Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet 2012;379:1245-55. [Crossref] [PubMed]
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50. [Crossref] [PubMed]
- Wang YY, Zhong JH, Su ZY, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. Br J Surg 2016;103:725-34. [Crossref] [PubMed]
- Motoyama H, Kobayashi A, Yokoyama T, et al. Liver failure after hepatocellular carcinoma surgery. Langenbecks Arch Surg 2014;399:1047-55. [Crossref] [PubMed]
- Pan QX, Zhang JH, Su ZJ, et al. The Glasgow Prognostic Score is an independent prognostic predictor of hepatocellular carcinoma following radical resection. Oncol Res Treat 2014;37:192-7. [Crossref] [PubMed]
- Fu YP, Ni XC, Yi Y, et al. A Novel and Validated Inflammation-Based Score (IBS) Predicts Survival in Patients With Hepatocellular Carcinoma Following Curative Surgical Resection: A STROBE-Compliant Article. Medicine (Baltimore) 2016;95:e2784. [Crossref] [PubMed]
- Gabriel E, Attwood K, Shah R, et al. Novel Calculator to Estimate Overall Survival Benefit from Neoadjuvant Chemoradiation in Patients with Esophageal Adenocarcinoma. J Am Coll Surg 2017;224:884-94.e1. [Crossref] [PubMed]
- Gabriel E, Attwood K, Thirunavukarasu P, et al. Predicting Individualized Postoperative Survival for Stage II/III Colon Cancer Using a Mobile Application Derived from the National Cancer Data Base. J Am Coll Surg 2016;222:232-44. [Crossref] [PubMed]
- Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 2000;56:337-44. [Crossref] [PubMed]
- Gory I, Fink M, Bell S, et al. Radiofrequency ablation versus resection for the treatment of early stage hepatocellular carcinoma: a multicenter Australian study. Scand J Gastroenterol 2015;50:567-76. [Crossref] [PubMed]
- Vitale A, Burra P, Frigo AC, et al. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol 2015;62:617-24. [Crossref] [PubMed]
- Huang YH, Wu JC, Chau GY, et al. Supportive treatment, resection and transcatheter arterial chemoembolization in resectable hepatocellular carcinoma: an analysis of survival in 419 patients. Eur J Gastroenterol Hepatol 1999;11:315-21. [Crossref] [PubMed]
- Huang J, Yan L, Cheng Z, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg 2010;252:903-12. [Crossref] [PubMed]
- Cucchetti A, Piscaglia F, Cescon M, et al. Systematic review of surgical resection vs radiofrequency ablation for hepatocellular carcinoma. World J Gastroenterol 2013;19:4106-18. [Crossref] [PubMed]
- Jin YJ, Lee JW, Choi YJ, et al. Surgery versus transarterial chemoembolization for solitary large hepatocellular carcinoma of BCLC stage A. J Gastrointest Surg 2014;18:555-61. [Crossref] [PubMed]
- Elnekave E, Erinjeri JP, Brown KT, et al. Long-term outcomes comparing surgery to embolization-ablation for treatment of solitary HCC<7 cm. Ann Surg Oncol 2013;20:2881-6. [Crossref] [PubMed]
- Maluccio M, Covey AM, Gandhi R, et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J Vasc Interv Radiol 2005;16:955-61. [Crossref] [PubMed]
- Hsu CY, Liu PH, Lee YH, et al. Aggressive therapeutic strategies improve the survival of hepatocellular carcinoma patients with performance status 1 or 2: a propensity score analysis. Ann Surg Oncol 2015;22:1324-31. [Crossref] [PubMed]
- Hsu CY, Liu PH, Lee YH, et al. Hepatocellular Carcinoma Patients With Performance Status 1 Deserve New Classification and Treatment Algorithm in the BCLC System. Medicine (Baltimore) 2015;94:e1223. [Crossref] [PubMed]
- Zhong JH, Ke Y, Gong WF, et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg 2014;260:329-40. [Crossref] [PubMed]
- Ha J, Yan M, Aguilar M, et al. Race/ethnicity-specific disparities in cancer incidence, burden of disease, and overall survival among patients with hepatocellular carcinoma in the United States. Cancer 2016;122:2512-23. [Crossref] [PubMed]
- Fan HL, Hsieh CB, Chang WC, et al. Advanced age is not a contraindication for liver resection in cases of large hepatocellular carcinoma. Eur J Surg Oncol 2014;40:214-9. [Crossref] [PubMed]
- Kassahun WT. Contemporary management of fibrolamellar hepatocellular carcinoma: diagnosis, treatment, outcome, prognostic factors, and recent developments. World J Surg Oncol 2016;14:151. [Crossref] [PubMed]
- Li MX, Zhao H, Bi XY, et al. Total tumor volume predicts survival following liver resection in patients with hepatocellular carcinoma. Tumour Biol 2016;37:9301-10. [Crossref] [PubMed]
- Cucchetti A, Cescon M, Ercolani G, et al. A comprehensive meta-regression analysis on outcome of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Ann Surg Oncol 2012;19:3697-705. [Crossref] [PubMed]
- Bigonzi E, Cucchetti A, Pinna AD. Meta-analysis of anatomic resection versus non-anatomic resection for hepatocellular carcinoma: are they comparing apples with oranges? Langenbecks Arch Surg 2012;397:141-2; author reply 142. [Crossref] [PubMed]
- Wong TC, Cheung TT, Chok KS, et al. Treatment strategy to improve long-term survival for hepatocellular carcinoma smaller than 5 cm: major hepatectomy vs minor hepatectomy. World J Surg 2014;38:2386-94. [Crossref] [PubMed]
- Yang T, Lu JH, Lau WY, et al. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: A Propensity Score Matching Analysis. J Hepatol 2016;64:583-93. [Crossref] [PubMed]
- Lee JW, Lee YJ, Park KM, et al. Anatomical Resection But Not Surgical Margin Width Influence Survival Following Resection for HCC, A Propensity Score Analysis. World J Surg 2016;40:1429-39. [Crossref] [PubMed]
- Harimoto N, Shirabe K, Ikegami T, et al. Postoperative complications are predictive of poor prognosis in hepatocellular carcinoma. J Surg Res 2015;199:470-7. [Crossref] [PubMed]
- Nault JC, De Reynies A, Villanueva A, et al. A hepatocellular carcinoma 5-gene score associated with survival of patients after liver resection. Gastroenterology 2013;145:176-87. [Crossref] [PubMed]
- Zhao WC, Zhang HB, Yang N, et al. Preoperative predictors of short-term survival after hepatectomy for multinodular hepatocellular carcinoma. World J Gastroenterol 2012;18:3272-81. [PubMed]
- Kudo A, Matsumura S, Ban D, et al. Does the preoperative alpha-fetoprotein predict the recurrence and mortality after hepatectomy for hepatocellular carcinoma without macrovascular invasion in patients with normal liver function? Hepatol Res 2014;44:E437-46. [Crossref] [PubMed]


