Safety and initial efficacy of radiation segmentectomy for the treatment of hepatic metastases
Introduction
Hepatic metastatic disease is common in many malignancies (1) and contributes to severe morbidity and mortality (2,3). Hepatic metastatectomy and thermal ablation are associated with prolonged survival and are sanctioned by multiple guidelines (4-6). Many lesions are not anatomically amenable to resection or thermal ablation (7,8) due to proximity to critical structures, tumor size, indeterminate disease biology, and insufficient residual functional liver.
Transarterial radioembolization (TARE) with Yttrium-90 microspheres has developed a versatile role in the treatment of both primary and metastatic liver disease which includes neo-adjuvant, curative, and palliative applications (9-11). Furthermore, TARE has been used to successfully treat hepatic metastases of multiple other origins including traditionally radioresistant disease such as renal cell carcinoma (12-14). Segmental ablative radioembolization, or radiation segmentectomy (RS), allows for both increased safety and efficacy when compared to lobar or whole liver treatment by allowing for increased dose and sparing of uninvolved parenchyma (15). Early evaluation of RS in hepatocellular carcinoma with multicenter liver explant and post-resection studies demonstrates 100% and 50–99% necrosis in 52% and 48% of patients and similar tumor control to microwave plus transarterial chemoembolization in a large, propensity score matched, retrospective study (16-20). Our goal was to retrospectively evaluate the safety and initial efficacy of RS as a definitive radiotherapy for hepatic metastases in patients who were poor surgical or thermal ablation candidates.
Methods
Institutional review board approval was obtained for this study. A retrospective chart review of patients with hepatic metastatic disease treated with RS from 8/2015–6/2017 was performed. Inclusion criteria for our patients included liver dominant metastatic disease to the liver confined to two or fewer segments within a single angiosome and denial of surgical intervention by a multidisciplinary tumor board. RS was defined as TARE administered to two contiguous segments or less. Safety parameters evaluated were pre and post-procedure liver chemistry, MELD score, ALBI grade, platelet count, and adverse events using CTCAE v. 4.0 (21) and CD (22) classifications. Yittrium-90 containing glass microspheres and MIRD dosimetry were utilized for all patients. Initial efficacy was evaluated using RECIST 1.1, mRECIST, and PERCIST criteria based on initial and available post-procedure imaging.
Patients underwent pre-procedure mapping angiography utilizing cone beam CT (CBCT) with technetium macroaggregated albumin (MAA) SPECT fusion (23). Dosimetry varied based on tumor to normal liver uptake with an intended administered dose of 120 Gy or greater to the targeted angiosome. All patients subsequently underwent single session outpatient treatment with RS. Follow-up imaging was performed with PET/CT, contrast enhanced CT, or contrast enhanced MRI at means of 1 [1–3], 4 [3–7], and 6 [5–8] months post treatment. Serologic evaluation was performed within the first 3 months with a range of 4–11 weeks. Given the variable primary disease origins and imaging characteristics RECIST 1.1, mRECIST, or PERCIST criteria were utilized to evaluate therapy response based on lesion pretreatment enhancement or FDG uptake.
Results
Ten patients with hepatic metastases (7 colorectal, 1 breast, 1 leiomyosarcoma, 1 carcinoid) underwent 1–3 RS treatments (Table 1). One patient underwent a RS which was technically unsuccessful due to attenuated vascular anatomy. A single patient had a CTCAE Grade 1/CD Grade 1 adverse event consisting of self-limited hiccups following a hepatic dome treatment which may have been secondary to phrenic nerve irritation. There was no clinically significant post-treatment change in AST (mean pre-treatment 28 IU/L, post-treatment 31 IU/L), ALT (mean pre-treatment 28 IU/L, post-treatment 29 IU/L), MELD score (mean pre-treatment 7.0, post-treatment 6.8), ALBI score (mean pre-treatment −2.76, post-treatment −2.62), or platelet count (mean pre-treatment 160,000/mm3, post-treatment 194,000/mm3) at a mean of 3 months. Mean angiosomal radiation volume and dose was 353 mL (47–722 mL) and 261 Gy (119–477 Gy), respectively.
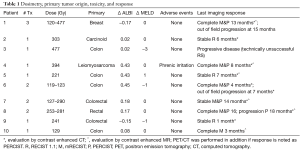
Full table
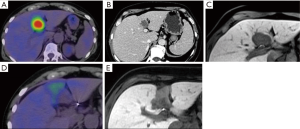
Initial efficacy post RS demonstrated PERCIST complete metabolic responses in five patients [colorectal, leiomyosarcoma, and breast (Figure 1), mean dose 269 Gy, 119–477 Gy], PERCIST partial metabolic response in one patient with one lesion (colorectal, dose 127 Gy), and RECIST 1.1 stable disease in four patients with four lesions (colorectal and carcinoid, mean dose 268 Gy and range, 221–303 Gy). One patient showed progression by mRECIST at 13 months requiring a second treatment (Rectal 1st dose 253 Gy, 2nd dose 281 Gy). Mean progression free survival for this heterogeneous cohort was 7.1 [1–16] months.
One patient who had a PERCIST partial response showed stable disease at 14 months. A single patient who had complete response in two separate lesions developed multifocal disease progression. Nine out of ten patients had systemic chemotherapy and none developed dose limiting hepatotoxicity.
Discussion
The role of TARE for the management hepatic metastases is currently being developed (24,25). Our results suggest that early evaluation of RS, despite administered doses in excess of 470 Gy (26), is a potentially safe treatment option for select patients with hepatic metastatic disease. Transarterial brachytherapy is unique in both the ability to safely deliver predictable and highly conformal ablative radiation doses in close proximity to vulnerable anatomy while allowing for repeat treatments without cumulative organ dose. Advances in stereotactic body radiation and proton therapy allow for hypofractionated treatments to select hepatic malignancies (27,28), but are subject to dose constraints, risk of injury to adjacent structures such as bowel, variability due to patient motion requiring fiducial placement, and limitations to repeat administration which are not present in RS.
Of interest, complete metabolic responses were obtained in lesions with minimal to no enhancement or Tc-MAA uptake. While pre-treatment enhancement does not always correlate with response, higher radiation doses conceptually generate higher mEv beta particle events which could mitigate radiation watershed. As RS may be performed as a single outpatient session (29), offering a minimally invasive definitive radiotherapy with little impact on quality of life and the potential to improve disease free progression is an appealing concept.
Future developments in this approach include dose escalation studies and the consideration for concurrent systemic radiosensitization. Another promising area of exploration is the possible potentiation of immunotherapy, radiotherapy has been shown to be a prolific immunomodulator (30,31).
There are multiple limitations to this study given its retrospective nature, small sample size with variable primary cancers, lack of pathologic correlation, variable imaging schedule and modalities and lack of long-term response data. Many of these factors are inherent to the limited population of patients with metastatic disease who are candidates for RS at this time. Ultimately, whether imaging responses translate to improved survival will require investigation.
Conclusions
Early evaluation of segmental transarterial radioembolization indicates a potentially safe treatment option for select patients with hepatic metastatic disease. Initial efficacy as definitive radiotherapy with minimal hepatic toxicity is promising, particularly in anatomic locations unamenable to resection or alternative means of ablation.
Acknowledgements
None.
Footnote
Conflicts of Interest: B Geller and B Toskich are consultants for BTG plc. The other authors have no conflicts of interest to declare.
Ethical Statement: IRB approval was obtained by the University of Florida (No. 201500230). All patients signed informed consent prior to treatment regarding the collection of treatment data.
References
- Leporrier J, Maurel J, Chiche L, et al. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br J Surg. 2006;93:465-74. [Crossref] [PubMed]
- Patanaphan V, Salazar OM. Colorectal cancer: metastatic patterns and prognosis. South Med J 1993;86:38-41. [Crossref] [PubMed]
- Riihimäki M, Hemminki A, Fallah M, et al. Metastatic sites and survival in lung cancer. Lung Cancer 2014;86:78-84. [Crossref] [PubMed]
- Guenette JP, Dupuy DE. Radiofrequency ablation of colorectal hepatic metastases. J Surg Oncol 2010;102:978-87. [Crossref] [PubMed]
- Frankel TL, D’Angelica MI. Hepatic resection for colorectal metastases. J Surg Oncol 2014;109:2-7. [Crossref] [PubMed]
- Sheth KR, Clary BM. Management of hepatic metastases from colorectal cancer. Clin Colon Rectal Surg 2005;18:215-23. [Crossref] [PubMed]
- Yang TX, Chua TC, Morris DL. Radioembolization and chemoembolization for unresectable neuroendocrine liver metastases - a systematic review. Surg Oncol 2012;21:299-308. [Crossref] [PubMed]
- Popescu I, Alexandrescu ST. Surgical options for initially unresectable colorectal liver metastases. HPB Surg 2012;2012:454026. [PubMed]
- Sato K, Lewandowski RJ, Bui JT, et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol 2006;29:522-9. [Crossref] [PubMed]
- Habib A, Desai K, Hickey R, et al. Transarterial approaches to primary and secondary hepatic malignancies. Nat Rev Clin Oncol 2015;12:481-9. [Crossref] [PubMed]
- Benson AB, Venook AP, Cederquist L, et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:370-98. [Crossref] [PubMed]
- Johnston FM, Mavros MN, Herman JM, et al. Local therapies for hepatic metastases. J Natl Compr Canc Netw 2013;11:153-60. [Crossref] [PubMed]
- Hickey R, Lewandowski RJ, Prudhomme T, et al. 90Y Radioembolization of Colorectal Hepatic Metastases Using Glass Microspheres: Safety and Survival Outcomes from a 531-Patient Multicenter Study. J Nucl Med 2016;57:665-71. [Crossref] [PubMed]
- Gaba RC, Lakhoo J. Yttrium-90 microsphere radioembolization for treatment of lung cancer hepatic metastases. Case Rep Oncol 2012;5:479-86. [Crossref] [PubMed]
- Riaz A, Gates VL, Atassi B, et al. Radiation segmentectomy: a novel approach to increase safety and efficacy of radioembolization. Int J Radiat Oncol Biol Phys 2011;79:163-71. [Crossref] [PubMed]
- Salem R, Gordon AC, Mouli S, et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared With Chemoembolization in Patients With Hepatocellular Carcinoma. Gastroenterology 2016;151:1155-63.e2. [Crossref] [PubMed]
- D’Avola D, Lñarrairaegui M, Bilbao JI, et al. A retrospective comparative analysis of the effect of Y90-radioembolization on the survival of patients with unresectable hepatocellular carcinoma. Hepatogastroenterology 2009;56:1683-8. [PubMed]
- Toskich BB, Tabriz DM, Zendejas I, et al. Transportal Radioembolization as Salvage Hepatocellular Carcinoma Therapy to Maintain Liver Transplant Candidacy. J Vasc Interv Radiol 2015;26:1479-83. [Crossref] [PubMed]
- Edeline J, Gilabert M, Garin E, et al. Yttrium-90 microsphere radioembolization for hepatocellular carcinoma. Liver Cancer 2015;4:16-25. [Crossref] [PubMed]
- Shah JL, Zendejas-Ruiz IR, Thornton LM, et al. Neoadjuvant Transarterial Radiation Lobectomy for Colorectal Hepatic Metastases: A Small Cohort Analysis on Safety, Efficacy, and Radiopathologic Correlation. J Gastrointest Oncol 2017;8:E43-51. [Crossref] [PubMed]
- National Institute of Cancer. Common Terminology Criteria for Adverse Events (CTCAE). NIH Publ 2010;9:1-71.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Gates VL, Singh N, Lewandowski RJ, et al. Intraarterial Hepatic SPECT/CT Imaging Using 99mTc-Macroaggregated Albumin in Preparation for Radioembolization. J Nucl Med 2015;56:1157-62. [Crossref] [PubMed]
- Padia SA, Lewandowski RJ, Johnson GE, et al. Radioembolization of Hepatic Malignancies: Background, Quality Improvement, Guidelines, and Future Directions. J Vasc Interv Radiol 2017;28:1-15. [Crossref] [PubMed]
- Kis B, Shah J, Choi J, et al. Transarterial Yttrium-90 Radioembolization Treatment of Patients with Liver-Dominant Metastatic Renal Cell Carcinoma. J Vasc Interv Radiol 2017;28:254-9. [Crossref] [PubMed]
- Kao JL, Bourgeois AC, Chang T, et al. How to determine absorbed dose following radioembolization using Y90 PET/CT: A clinician’s guide. J Vasc Interv Radiol 2014;25:S162-3. [Crossref]
- Sugahara S, Oshiro Y, Nakayama H, et al. Proton beam therapy for large hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2010;76:460-6. [Crossref] [PubMed]
- Wigg A, Hon K, Mosel L, et al. Successful downstaging of hepatocellular carcinoma using of external beam radiotherapy with subsequent liver transplantation: Proof of principle. J Gastroenterol Hepatol 2012;27:94. [PubMed]
- Gabr A, Kallini J, Gates V, et al. Same-day Y90: pretreatment mesenteric angiography, 99mTc-MAA scan, and Y90 radioembolization in a single outpatient encounter. J Vasc Interv Radiol 2016;27:S156. [Crossref]
- Soukup K, Wang X. Radiation meets immunotherapy - a perfect match in the era of combination therapy? Int J Radiat Biol 2015;91:299-305. [Crossref] [PubMed]
- Sgambato A, Casaluce F, Sacco PC, et al. Anti PD-1 and PDL-1 Immunotherapy in the Treatment of Advanced Non- Small Cell Lung Cancer (NSCLC): A Review on Toxicity Profile and its Management. Curr Drug Saf 2016;11:62-8. [Crossref] [PubMed]


