Tumor location impact in stage II and III colon cancer: epidemiological and outcome evaluation
Introduction
Colon cancer (CC) is a public health problem worldwide and in Tunisia. The incidence is estimated to be 1.2 million per year, and more than 600,000 deaths every year (1). In the recent years, with knowledge advancement in genetic and molecular mechanism of carcinogenesis, CC is no longer considered as a unique disease. Distinguishing CC based on anatomical location was first described by Bufill et al. in 1990 (2). Subsequent publications pointed out several differences between right-sided (RCC) and left-sided CC (LCC) regarding epidemiology, pathogenesis, embryologic, genetic-epigenetic alterations, molecular pathways and outcome (2-4). Roughly, it is suggested that anatomical site could have, in the future, an impact in the management of CC. However, data regarding prognosis remain controversial and a great debate is open whether tumor location itself plays a prognostic role. A poorer survival of RCC was reported by most studies (5,6). These observations are more evident in advanced stage with differences in response to targeted therapies (7,8). In a large population based study of 57,847 patients from the surveillance, epidemiology and end results (SEER) database, disease specific survival was significantly worse in RCC patients vs. LCC patients, with a hazard ration of 0.77 (0.72±0.81) in stage IV (4). However, in early stage data are less defined and prognostic role is still under investigation. Patterns of such anatomical distribution in various population would help a better understanding of this issue. We aimed in our current study to describe clinico-pathological characteristics and differences between RCC and LCC in Tunisian population. We also analyzed outcome to determine whether location is of prognostic significance.
Methods
We retrospectively analyzed a cohort of 203 patients, with histologically confirmed, stage II and III CC with complete work-up and treated with curative intent during the period 2003–2014.The TNM staging system of the American Joint Committee on Cancer (AJCC/UICC 7th edition) was used for staging. Only adenocarcinoma and CC cases were included. We considered two groups: RCC and LCC. The right counterpart or RCC was defined as the colon sections going from the appendix-cecum, ascending colon, hepatic flexure and transverse colon to the left angle before the limit of the splenic flexure. LCC was defined as the sections going from the splenic flexure, descending colon, the sigmoid until the recto-sigmoid junction. If tumors were cited in both left-sided and right-sided locations or the origin could not be ascribed to either side, the patient was excluded from the present analysis. Clinico-pathological data including: age at diagnosis, gender, medical history, tumor location, histological type, grade/stage of tumor, chemotherapy history were collected from patients records and were compared between RCC and LCC. We analyzed outcome parameters were studied: overall survival (OS), relapse free survival (RFS) for both groups. We also reported annual hazard of relapse (AHR) for RCC vs. LCC during the first 4 years of follow up.
Statistical analysis
Statistical analysis was performed using the statistical package for the social sciences (SPSS) 16.0. Comparison of variables was performed using Pearson’s chi-square test, Fisher’s exact test. OS was defined as the time from first therapeutic action to death from any cause or loss to follow up or latest news. RFS was defined as the time from first therapeutic action to first recurrence confirmed by radiological of histological feature or death. Survival analyses were done through a Cox proportional hazard function for both univariate and multivariate analyses, and Kaplan-Meier (log-rank test) curves were plotted. Significance for all statistics were recorded if P<0.05. Annual relapse rate was defined as the fraction of followed patients who had recurring disease in a 1-year period restricted to follow-up contribution of each specified time interval.
Looking to the retrospective character of this study, no written informed consent was necessary from patients. Local ethical committee approved the study protocol, which was in accordance with the principles of the Helsinki Declaration.
Results
Overall population
We collected a cohort of 203 patients. Mean age was 58 years ranging from 27 to 85 and sex-ratio was 1.2 with 114 (56.2%) of the patients were male. Family history of colorectal cancer was observed in 32 (15.8%) of patients. Almost all patients (95.6%, 194) had body CT scan for work-up. Surgery was performed for surgical emergency in 15.8% (32 cases: 30 for bowel obstruction and 2 for peritonitis) during which 29 had upfront surgical resection of the tumor. All patients had anatomical tumor resections adapted to tumor location, except for 11 patients who had total colectomy. Pathology showed poorly differentiated tumors in 13 (6.4%), moderately differentiated tumors in 24 (11.8%), and well differentiated tumors in 101 (49.8%), differentiation being not defined in 65 (32%) cases. TNM staging showed: pT2 in 5 (2.5%) of cases, pT3 in 108 (53.2%), pT4 in 44.3% (90 cases). Nodal involvement/stage III disease was observed in 56.7% (115 patients), among them pT4 stage represented 46.1% (53 patients) and pN2 39.1% (45/115). In stage II patients, pT4 was seen in 42.1% (37/88 patients). Vascular emboli were seen in 24.6% (50 patients) and peri neural invasion in 37 (18.2%). Mean tumor size was 7.6 cm. Adjuvant chemotherapy was indicated in 178 (87.7%) of the patients; 119 (66.9%) received Folfox regimen and 59 (33.1%) received 5-FU based regimen, almost all patients finished 6 cycles of adjuvant therapy (98.8%). Relapse rate was 32.5% (66 cases). Median follow up was 49 months. We observed 150 (73.9%) patients with LCC and 53 (26.1%) patients with RCC. Characteristics of the two groups are described in Table 1. We did not observe a statistically significant difference in terms of age, tumor stage and therapy between both groups. RCC patients were significantly more likely to be female then LCC patients (56.6% vs. 39.3%, P=0.029). RCC tumors were also significantly more likely to be undifferentiated then LCC (87.1% vs. 8.4%, P=0.014).
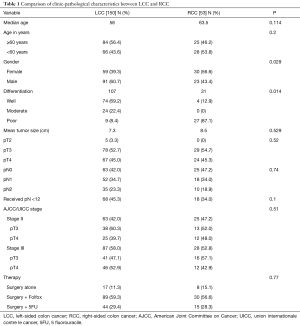
Full table
At the last time to follow up, 85.3% of LCC patients were still alive and 73.6% RCC were still alive. Five-year OS was significantly worse in RCC vs. LCC (65% vs. 82%, hazard ratio (HR) =2.07; 95% CI: 1.05–4.09; P=0.03) (Figure 1). There was no difference in relapse free survival between the groups (HR =1.15; 95% CI: 0.66–2.19; P=0.6) (Figure 2). Median time to relapse was significantly shorter in RCC (15 months) vs. LCC (24 months), P=0.005. Recurrence rate was 32% in both groups, P=0.9. In subgroup analysis, there was no impact of tumor location on survival for stage II disease, 5-year OS was 78% in RCC vs. 82% in LCC (HR =1.94; 95% CI: 0.54–6.93; P=0.29). However, tumor location significantly impacted survival in stage III disease, 5-year OS was 45% in RCC, and 63% in LCC (HR =2.28; 95% CI:1.01–5.24; P=0.04). During the interval first-second year to follow up, we observed an annual relapse rate peak of 15.6% in RCC group vs. 3.2% in LCC with a significant P value, P=0.026. We did not observe a significant difference in annual relapse rate between the groups in the other year-intervals of follow up.
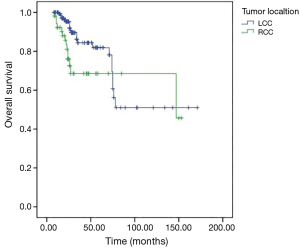
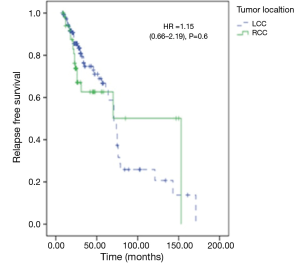
Discussion
Our study showed differences in clinic-pathological and outcome characteristics between RCC and LCC locations. Patients with RCC were more likely to be female, with poorly differentiated tumors and worse survival, especially in stage III. RCC patients tended to relapse earlier with a peak in the first-second year to follow-up. Our results go along with results of previous studies. Differences between both anatomic sides of the colon can be due to numerous reasons: different embryological origin, different microbiota, different genetic pathways…These differences translate into the clinical presentation; the incidence of RCC is associated with a number of risk factors, female gender, old age, previous cancer history, and insulin resistance, while LCC is related to individuals with a low fiber diet, heavy smokers, and alcohol consumers (9). In comparison with LCC, RCC has a prevalence toward being poorly-differentiated, commonly mucinous histology type, a more advanced disease and often involving satellite the lymph nodes or peritoneal region rather than the liver or lung, which are the most common sites of metastasis from left CC (7).
Exact anatomical definition of RCC and LCC is still unclear, for now the consensus is to consider the splenic flexure as the cut-off limit. In Meguid et al. study; analysis of anatomic stratification showed that mortality risk was 8.0% greater in case of location between hepatic flexure and splenic flexure compared with those having a tumor located between the descending colon and sigmoid colon (HR 1.08; 95% CI: 1.05–1.11; P<0.001). Subjects with cancer between the cecum and ascending colon had a 3.7% greater mortality risk compared with those with cancer between the descending colon and sigmoid colon (HR 1.037; 95% CI: 1.01–1.06; P=0.007) (10). Prognostic significance was reported by several studies; however, the implication on our current practice is still unclear. Should we change our decisions in regard to indication of adjuvant therapy or of particular regimens based on tumor location? This is a question without a clear answer. The predictive value on survival in metastatic setting was confirmed in many clinical trials (11-13). It seems that left-sided primary tumor site is a useful predictor of improved cetuximab efficacy in RAS wild-type metastatic colorectal cancer, but patients with right-sided RAS and BRAF wild-type metastatic colorectal cancer seemed to derive no benefit from single-agent anti-EGFRs. Upfront comparison between cetuximab and bevacizumab in the FIRE3 study reported similar observations (8,14). It is although important to highlight that all reported studies are retrospective and post hoc unplanned analyses with unbalanced groups and heterogenous definition of tumor side. There is an urgent need to perform clinical trials with pre stratified subgroups to change our clinical practice guidelines. Data in early stage CC are very conflicting. Four published large population cohorts from the SEER data based including patients treated in different periods of time (Table 2) showed different results. First, in the study of Meguid et al. differences in survival between right- and left-sided CC with stage I were observed (HR =1.003; P=0.93) and stage II right-sided colon cancers had lower HR than those with left-sided colon cancers (HR =0.91; P<0.001) (10). In 2008, Weiss et al. reported, in a mortality analysis of 53,801 patients with stage I–III CC, no significant difference in mortality between right and left-sided cancers for all stages combined (HR =1.01; 95% CI: 0.98–1.04; P=0.598) and not for stage I cancers (HR =0.95; 95% CI: 0.88–1.03; P=0.211). However, stage II right-sided cancers had lower mortality than left-sided cancers (HR =0.92; 95% CI: 0.87–0.97; P=0.001), and stage III right-sided cancers had higher mortality (HR =1.12; 95% CI: 1.06–1.18; P=0.001) (5). Recently Yang et al. demonstrated that among stages I and II disease, RCC patients had better disease specific survival than those with LCC. However, among stages III, outcome was worse (4). And finally, Warschkow et al. reported that in stage I and II, the prognosis of right-sided cancer was better for overall (HR =0.89, 95% CI: 0.84–0.94 and HR =0.85, 95% CI: 0.81–0.89) and cancer-specific survival (HR =0.71, 95% CI: 0.64–0.79 and HR =0.75, 95% CI: 0.70–0.80). Right- and left-sided CC had a similar prognosis for stage III (overall: HR =0.99, 95% CI: 0.95–1.03 and cancer-specific: HR =1.04, 95% CI: 0.99–1.09) (15).

Full table
In our study, tumor location had impact only in stage III CC. This heterogeneity in reported survival results is due to tumor heterogeneity. In fact, tumor cells with specific genotype in a single tumor, may present variations in response to environment and treatments phenotypes and may change with process of tumor infiltration and metastasis (16,17).
Therapeutic implications in early stage CC of tumor location are now described in several series. Benefit from adjuvant chemotherapy in stage III CC, could be deeper in RCC (HR =0.37; P<0.0001) as reported by Elsaleh et al. (18). However in the N0147 trial, survival benefit from adjuvant Folfox in stage III was greater in LCC, this benefit was different between RCC and LCC when mismatch repair status was considered (19). Folfiri which is not a standard of care in the adjuvant setting, was reported to be more effective in stage II RCC then in LCC, in a retrospective analysis of the PETACC3 adjuvant trial (20).
In conclusion, considering tumor location is an important factor for future studies about early and advanced CC. It represents a future possible field into deeper personalized medicine in colorectal cancer (CRC) management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol was approved by the review board of Abderrahmen Mami Hospital, which is affiliated to Faculty of Medicine Tunis (FMT) (décret n° 94-1939 du 19 septembre 1994).
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med 1990;113:779-88. [Crossref] [PubMed]
- Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer 2002;101:403-8. [Crossref] [PubMed]
- Yang J, Du XL, Li ST, et al. Characteristics of differently located colorectal cancers support proximal and distal classification: a population-based study of 57,847 patients. PLoS One 2016;11:e0167540. [Crossref] [PubMed]
- Weiss JM, Pfau PR, O’Connor ES, et al. Mortality by stage for right-versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol 2011;29:4401-9. [Crossref] [PubMed]
- Suttie SA, Shaikh I, Mullen R, et al. Outcome of right- and left-sided colonic and rectal cancer following surgical resection. Colorectal Dis 2011;13:884-9. [Crossref] [PubMed]
- Shen H, Yang J, Huang Q, et al. Different treatment strategies and molecular features between right-sided and left-sided colon cancers. World J Gastroenterol 2015;21:6470-8. [Crossref] [PubMed]
- Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol 2016. [Epub ahead of print]. [PubMed]
- Xiang L, Zhan Q, Zhao XH, et al. Risk factors associated with missed colorectal flat adenoma: a multicenter retrospective tandem colonoscopy study. World J Gastroenterol 2014;20:10927-37. [Crossref] [PubMed]
- Meguid RA, Slidell MB, Wolfgang CL, et al. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol 2008;15:2388-94. [Crossref] [PubMed]
- Wong HL, Lee B, Field K, et al. Impact of primary tumor site on bevacizumab efficacy in metastatic colorectal cancer. Clin Colorectal Cancer 2016;15:e9-15. [Crossref] [PubMed]
- Moretto R, Cremolini C, Rossini D, et al. Location of primary tumor and benefit from anti-epidermal growth factor receptor monoclonal antibodies in patients with RAS and BRAF wild-type metastatic colorectal cancer. Oncologist 2016;21:988-94. [Crossref] [PubMed]
- Chen KH, Shao YY, Chen HM, et al. Primary tumor site is a useful predictor of cetuximab efficacy in the third-line or salvage treatment of KRAS wild-type (exon 2 non-mutant) metastatic colorectal cancer: a nationwide cohort study. BMC Cancer 2016;16:327. [Crossref] [PubMed]
- Holch JW, Ricard I, Stintzing S, et al. The relevance of primary tumour location in patients with metastatic colorectal cancer: a meta-analysis of first-line clinical trials. Eur J Cancer 2017;70:87-98. [Crossref] [PubMed]
- Warschkow R, Sulz MC, Marti L, et al. Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer 2016;16:554. [Crossref] [PubMed]
- Bretthauer M, Kaminski MF, Løberg M, et al. Population-based colonoscopy screening for colorectal cancer: a randomized clinical trial. JAMA Intern Med 2016;176:894-902. [Crossref] [PubMed]
- Jayasekara H, Reece JC, Buchanan DD, et al. Risk factors for metachronous colorectal cancer following a primary colorectal cancer: A prospective cohort study. Int J Cancer 2016;139:1081-90. [Crossref] [PubMed]
- Elsaleh H, Joseph D, Grieu F, et al. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet 2000;355:1745-50. [Crossref] [PubMed]
- Sinicrope FA, Mahoney MR, Smyrk TC, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol 2013;31:3664-72. [Crossref] [PubMed]
- Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25:1995-2001. [Crossref] [PubMed]


