Disparities in resection of hepatic metastases in colon cancer
Introduction
Current literature supports the safety and efficacy of hepatic resection for metastatic colorectal cancer (1,2). While colorectal cancer (CRC) has been decreasing over recent years in older patients (3), there has been an overall increase in rates of liver resections performed for metastatic disease, particularly those from colorectal cancer (4). Furthermore, favorable outcomes have been reported, with 5-year survival rates following hepatic metastasectomy ranging from 24–58% (5-9) and 10-year survival rates of approximately 25% (5-7). There are limited data however, describing national population trends of patients with stage IV colon cancer undergoing resections for synchronous hepatic metastases (SHM). Furthermore, there is no information about patient-level differences in the use of this aggressive surgical strategy.
Among colorectal cancer patients, certain populations are at higher risk of developing disease, specifically Alaskan Natives and African Americans (10). African American patients are also more likely to present with advanced disease and have lower 5-year survival rates compared to Caucasian counterparts (11,12). Additionally, African American patients are less likely to undergo minimally invasive surgery than either white or Asian patients, and are at higher risk for readmission and mortality following open surgery (13). While outcomes studies have been conducted in specific cohorts (14,15), literature evaluating the utilization of hepatic resection for metastatic colon cancer in certain demographic populations remains limited. Furthermore, mortality for certain cancers varies widely across US counties, suggesting the importance of factors including access to care, medical education, and socioeconomic status (16).
We sought to describe trends in resection rates of SHM in patients with stage IV colon cancer using a large national cohort database. Additionally, we aimed to identify significant factors associated with the likelihood of undergoing resection for metastatic disease. Among patients with stage IV colon cancer who did not undergo resection, we sought to characterize temporal trends and disparities in surgical evaluation.
Methods
We obtained data from the Surveillance, Epidemiology and End Results (SEER) Medicare files for patients diagnosed with Stage IV colon adenocarcinoma between 2000 and 2011 (17). Because they lacked complete claims data, we excluded patients from the study who were enrolled in HMOs or were not enrolled in Medicare Part A/B, within the prior 12 months or any time during follow-up. Patients older than 99 years as well as those 65 and younger were also excluded.
Identification of important events, including diagnosis of SHM, receipt of liver resection, and evaluation by a surgeon, was based on the presence of claims that occurred within 6 months prior to and 12 months after the date of cancer diagnosis for each patient. We identified patients who had evidence of SHM based on the presence of ICD-9 (International Classification of Diseases, 9th Revision) diagnosis codes (155.2 or 197.7) for secondary liver metastases.
Primary outcome was receipt of liver resection based on ICD-9 and Current Procedural Terminology (CPT) codes during the same time period. Procedures identified included laparoscopic liver resection (CPT 47370), wedge resection (CPT 47120, ICD-9 50.22), hepatic lobectomy (CPT 47125, 47130; ICD-9 50.3), and hepatic trisegmentectomy (CPT 47122). Radiofrequency ablation and cryoablation were not included as the purpose of this study primarily focused on surgical resections for definitive management of SHM. We also identified the receipt of colon resection during the same time period based on ICD-9/CPT codes. Colon resection was classified as elective or urgent/emergent based on the admission type found in the Medicare claim file associated with the identified colon procedure.
Secondary outcome was surgeon evaluation in the peri-diagnostic window in patients who did not ultimately receive either colon or liver resection. Evaluation by a surgeon was determined by the presence of CPT/HCPCS codes for office visits (99201-99215; 99214-99245) with an associated surgical specialization code (HCFASPEC 02, 28, 33, 77, 78, 91).
Prior to analysis, we defined the categorical variables we hypothesized would be associated with receipt of hepatic resection including patient age, race, gender, Klabunde-Charlson comorbidity score (18,19), socioeconomic status (based on % poverty level), urban/rural location, and year of diagnosis. Patient comorbidity was assessed using the Klabunde modification of the Charlson comorbidity score (CCS) (18,20) represented by three categories: 0 (low), 1 (moderate), and 2 or greater (high). The percentage of people living below the poverty line in a patient’s Census tract was used as a proxy for socioeconomic status. This was categorized as <5%, 5–10%, 10–20% and >20% below the poverty line. The urban/rural location was based on the codes provided in the SEER PEDSF file and was categorized as follows: metropolitan area (population ≥1 million), metropolitan area (population <1 million), and non-metropolitan.
Statistical analysis
Univariate associations between receipt of hepatic resection and the above-mentioned characteristics were assessed using Pearson’s χ2 tests for categorical variables. The adjusted association between the patient variables and hepatic resection were analyzed using a multivariable logistic regression including all variables significantly associated with hepatic resection in univariate analysis (P<0.05). A similar analysis was performed to evaluate the association between patient factors and surgeon evaluation among patients who never underwent any surgical therapy (either colon or liver resection).
Joinpoint regression analysis
We also utilized joinpoint regression to further examine specific temporal trends in liver resection and surgical evaluation for stage IV colon cancer patients (21). This analysis allows for calculation and plotting of best-fit regression models for the function of a dependent variable (i.e., hepatic resection) as it relates to a continuous independent variable (i.e., time). When the slope of the regression curve, which represents the trend over that time period, changes significantly from the slope of neighboring time intervals, a “joinpoint” is generated (22). Each interval change in the dependent variable over time is represented by the annual percentage change (APC). The overall change in the variable across the study period is the average annual percentage change (AAPC). When there are no joinpoints defined across a regression curve, the APC is equal to the AAPC. Trends are deemed statistically significant (P<0.05) when the AAPC is different from zero and the 95% confidence interval (95% CI) does not contain zero and the null hypothesis is rejected.
Two-tailed tests were used for all analyses and statistical significance was defined at P<0.05. Statistical analysis was conducted using STATA 14.0 (STATA Inc., College Station, TX, USA) and the Joinpoint Regression Program (version 4.4.0.0—June 2016; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute, Bethesda, MD). The study was reviewed and exempted by the Institutional Review Board of the Hospital of the University of Pennsylvania.
Results
Cohort characteristics
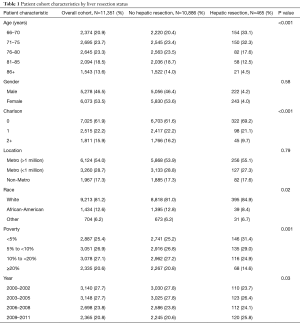
From 2000 to 2011, there were 11,351 patients with colon cancer and SHM (Table 1). Median age was 77 years (IQR 71, 82) with 83.3% (n=9,456) aged 70 or older as shown in Table 1. The majority of patients were female (n=6,073; 53.5%) and Caucasian (n=9,213; 81.2%). Mean Charlson Comorbidity Score was 0.66 with the majority (62%) having no reported comorbidities, 22% with 1, and 16% with 2 or more.
Full table
Hepatic resection
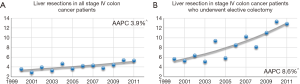
Among all cohort patients, only 465 (4.1%) underwent a surgical hepatic resection. The rate of resections per year increased significantly rising from 3.6% of stage IV patients with SHM in 2000 to 5.4% in 2011 (P=0.03). This trend was significant on joinpoint analysis [AAPC of 3.9% (95% CI: 1.6–6.4%, P<0.001)] as shown in Figure 1A. Among patients who underwent elective colectomies, the rate of hepatic resection was higher overall and increased at an even greater rate, rising from 5.7% in 2000 to 12.9% in 2011 (AAPC 8.6%, 95% CI: 5.2–12.2%, P<0.001) (Figure 1B).
Relative to patients not receiving hepatic resection, those who received hepatic resection were more likely to be younger, Caucasian, and have fewer comorbidities (Table 1). Almost 7% of the patients aged 66–70 underwent hepatic resection (33.1% of all patients who underwent resection), compared with 1.4% of patients older than 85 (4.5% of resection patients) (P<0.001). Patients with higher comorbidity scores were less likely to undergo hepatic resection, with only 2.5% of those patients with CCS of ≥2 undergoing resection, compared to 4.6% of those with scores of 0 (P<0.001).
Differences were also noted based on race and socio-economic status. Among Caucasian patients in the cohort, 4.3% underwent hepatic resection, compared to only 2.7% of African-American patients (P=0.02). Among patients living in areas with <5% poverty, 5.1% underwent hepatic resection, compared to 2.9% of patients living in areas with >20% poverty (P=0.001).
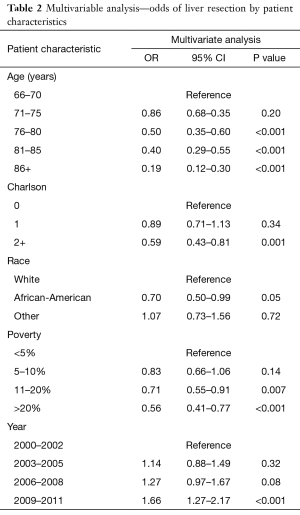
In multivariable analysis, age, comorbidity, race, poverty level, and calendar year were independent predictors of liver resection (Table 2). For example, patients age 76–80 were half as likely to undergo hepatic resection as those aged 66–70 (OR 0.50; 95% CI: 0.35–0.60; P<0.001). Similarly, likelihood of patients with a Charlson ≥2 undergoing hepatic resection was over 40% lower than those of patients with a Charlson score of 0 (OR 0.59; 95% CI: 0.43–0.81; P=0.001).
Full table
Significant disparities related to race and socioeconomic status were also identified. The odds of African American patients undergoing hepatic resection was 30% lower than those of Caucasian patients (OR 0.70; 95% CI: 0.50–0.99; P=0.05). Living in areas with higher poverty was also associated with a steady decrease in the odds of hepatic resection. Patients from areas with 10-20% or >20% poverty were almost 29% (OR 0.71; 95% CI: 0.55–0.91; P=0.007) and 44% (OR 0.56; 95% CI: 0.41–0.77; P<0.001) less likely to have a hepatic resection than patients from areas with <5% poverty.
Surgical evaluation
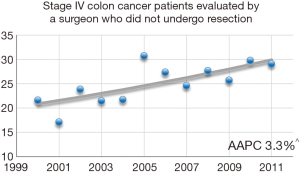
Rates of evaluation by a surgeon among patients who had no surgical therapy (colon or hepatic resection) were identified. Approximately 35% (n=3,984) of patients identified with SHM did not undergo colon or hepatic resection. Among these patients, 1,000 (25.1%) were evaluated by a surgeon in the peri-diagnosis period. Figure 2 demonstrates a significant increase in the proportion of patients receiving surgical evaluation over time from 21.6% in 2000 to 29.1% in 2011 (AAPC 3.3%, 95% CI: 1.1–5.6, P<0.001).
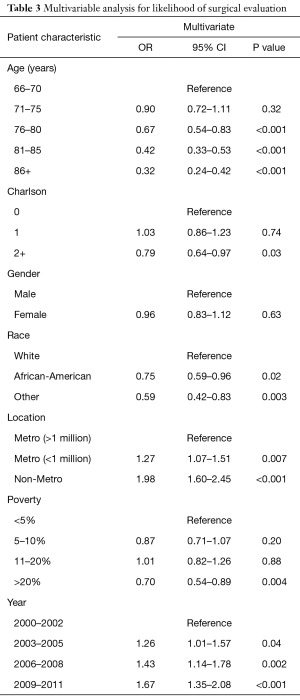
In multivariable analysis, several patient factors were significantly associated with surgical evaluation (Table 3). As with hepatic resection, the odds of surgical evaluation decreased steadily with increasing age and comorbidity. The odds of a 76–80-year old patient seeing a surgeon were 33% lower than those of a 66–70-year old patient (OR 0.67, P<0.001). The odds ratio for patients 81–85 years old was 0.42 (P<0.001). Patients with Charlson ≥2 had odds that were over 20% lower (OR 0.79, P=0.03) than patients with Charlson of 0.
Full table
Race and socioeconomic variables were also related to receipt of surgical evaluation. The odds of an African American seeing a surgeon were 25% lower than those for a Caucasian patient (OR 0.75, P=0.02). Patients from areas of highest poverty had significantly lower odds of a surgeon than those with the least poverty (OR 0.70, P=0.004). Patients from non-metropolitan areas had the highest odds of seeing a surgeon (OR 1.98, P<0.001). Patients from smaller metropolitan regions (<1 million) were also more likely to see a surgeon than those patients from the largest metropolitan areas (>1 million) (OR 1.27, P=0.007).
Discussion
This is a retrospective study of a national cohort of patients with stage IV colon cancer presenting with SHM between 2000–2011. Our findings demonstrate that, although rates of hepatic resection in this population remain low, they have increased steadily over time. We identified important disparities in the receipt of hepatic resection; namely African Americans and patients from high poverty locations were less likely to undergo hepatic resection. Similarly, we found that relatively few patients in the cohort actually saw a surgeon after diagnosis and that similar disparities exist.
It is important to note the overall low rate of hepatic resection in this cohort. Even in 2011, only 13% of these patients who received elective colon resection also had an identified hepatic resection. It is recognized that the incidence rate for colon cancer has been decreasing recently (23,24), likely due to increased efficacy in screening modalities and improved compliance with screening guidelines (25,26). Operations for patients with metastatic colon cancer, however, have been increasing as the safety profiles of these operations have improved and evidence has emerged supporting improved survival outcomes with resection of certain metastases (23,27-30). Our study confirms a trend toward a more aggressive surgical approach, specifically in patients with SHM, which has been demonstrated in previous studies (31,32). The overall low rates of hepatic resection in our cohort are paralleled by a low rate of surgical evaluation in the months surrounding diagnosis of stage IV colon cancer.
We also observed that older patients with more comorbidities were less likely to undergo hepatic resection. This is intuitive, as life expectancy for older patients may be less than that of the potential survival benefit that surgery may offer. Decreased quality of life and increased risk of morbidity in older patients with comorbidities may be a significant deterrent for those patients, their families, and their physicians when considering a major operation (33,34).
More concerning, however, is our finding that African American patients and patients from areas with higher poverty rates are significantly less likely to be seen by a surgeon and undergo hepatic resection. The explanation for this is likely multi-factorial, but all are important to consider. First, there could be provider-level differences in referring stage IV patients for surgical evaluation. It has been shown that race and socioeconomic status influence provider recommendations for invasive procedures (35,36). Also, these patients may be less likely to seek out surgical evaluation on their own if it is not offered by their primary treating physicians (37).
Second, African American patients and poorer patients may be less likely to accept surgical evaluation or consent to major cancer surgery. Minority patients hold more fatalistic views about the disease and are more likely to refuse recommended treatment (38). Education by providers regarding the safety of cancer surgery and evidence-based survival benefits, in the appropriate setting, are important to help increase uptake of cancer-directed therapy in these minority cohorts.
Finally, African American patients and poorer patients may receive lower rates of hepatic resection because they present more frequently with truly unresectable disease. It is well known that both racial and socioeconomic disparities exist in CRC screening and with stage of presentation of CRC. For example, Africans Americans are more likely than Caucasians to present with stage IV CRC (12). It is probable that disparities in screening and early identification of CRC contribute to this. Access to care and continuity of care are major factors in provision of adequate cancer screening as described in recent literature (39).
Several limitations of this study are worth mentioning. First, the analysis is limited to patients aged 65 years and above. However, more than half of CRC patients are diagnosed after the age of 65, making our findings generalizable to the diseased population. Also, the data available within this SEER-Medicare cohort do not allow for evaluation of disease-specific factors, which may play a role in surgical; decision-making. Most importantly, we cannot know the extent of tumor burden of the metastases at the time of presentation. Some proportion of patients who did not undergo surgical evaluation or resection may have been deemed, appropriately, to have unresectable disease at initial assessment. Additionally, we cannot determine the true rate of referrals for surgical evaluation by primary providers or recommendations for surgical therapy by surgeons. Furthermore, given the somewhat surprising low rates of surgical resection within the SHM cohort as well as surgical evaluation among those who did not undergo resection, errors in coding (i.e., miscoding of procedures or offices visits, and incomplete charting) must be considered. If, however, none of the above-mentioned differences existed, then the rates of resectable disease and surgical referrals, etc., should be similar across patient groups. That we see a significant difference in receipt of surgical evaluation and hepatic resection indicates that disparities likely exist.
Importantly, although we know the patients here presented with stage IV disease as coded by the SEER abstractor, we cannot verify that they had liver metastases. In fact, SEER-Medicare reviewers have substantive concerns about using these data to identify sites of metastases. We recognize that the SEER-Medicare reviewers feel any findings from this analysis may be inaccurate or misleading. We believe, however, that we addressed this by only including patients if an ICD-9 code for secondary liver malignancy was present in the peri-diagnosis time period. By only including patients who had an identifiable code, we feel that these patients represent a clean cohort of stage IV colon patients with hepatic metastases at the time of diagnosis. Additionally, as mentioned above, any under-diagnosis, or misdiagnosis, of metastatic disease site by coding, should be equal across groups and would not affect the important disparities in care noted here.
Conclusions
Resection for SHM in stage IV colon cancer can be safe and improve long-term outcomes. Consistent with this, we demonstrate that rates of surgical evaluation and hepatic resection in these patients have increased. The rates, however, remain low. Education should be provided to primary care providers and oncologists regarding the surgical options available, and their safety, even in patients with extensive liver disease. These providers should lower their thresholds for referring patients for surgical consultation, as lack of evaluation will result in lack of access to all possible treatment options.
More troubling is the evidence that significant patient-level differences in surgical evaluation and treatment continue to exist. It is imperative to identify modifiable barriers in access to evaluation and intervention in minority and poor patients. These barriers may exist as early as the screening process and extend all the way to provider and patient attitudes towards aggressive surgical therapy after diagnosis. In a recent article, Morris et al. address the multi-factorial issues surrounding disparities in cancer treatment (40). They offer several solutions including “dissemination and promotion of basic standards for quality of cancer care among all providers.” In stage IV disease, provider education regarding the available surgical treatment options and standardization of surgical referrals may help decrease the observed disparities. Additionally, continued patient education is required in the community to increase screening uptake, decrease the rate of unresectable disease, and increase the willingness of patients to undergo surgical evaluation and treatment. As pointed out by others, only education and policy changes based on recognized cultural differences will have a chance at addressing the disparities in cancer patients in “seeking, accepting, and receiving care.” (40).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was reviewed and exempted by the Institutional Review Board of the Hospital of the University of Pennsylvania (No. 00004028, Protocol #826611). A waiver of HIPAA authorization was obtained during the application and approval process for the study protocol.
References
- Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg 2003;197:233-41; discussion 241-2. [Crossref] [PubMed]
- Dulundu E, Attaallah W, Tilki M, et al. Simultaneous resection for colorectal cancer with synchronous liver metastases is a safe procedure: Outcomes at a single center in Turkey. Biosci Trends 2017;11:235-42. [Crossref] [PubMed]
- Siegel RL, Fedewa SA, Anderson W, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst 2017;109. [Crossref] [PubMed]
- de Ridder JA, Lemmens VE, Overbeek LI, et al. Liver Resection for Metastatic Disease; A Population-Based Analysis of Trends. Dig Surg 2016;33:104-13. [Crossref] [PubMed]
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25:4575-80. [Crossref] [PubMed]
- Chua TC, Saxena A, Chu F, et al. Predictors of cure after hepatic resection of colorectal liver metastases: an analysis of actual 5- and 10-year survivors. J Surg Oncol 2011;103:796-800. [Crossref] [PubMed]
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759-66. [Crossref] [PubMed]
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-25; discussion 825-7. [Crossref] [PubMed]
- Mavros MN, de Jong M, Dogeas E, et al. Impact of complications on long-term survival after resection of colorectal liver metastases. Br J Surg 2013;100:711-8. [Crossref] [PubMed]
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Chan C, Lopez A, Castaneda G, et al. Black Patients with Colorectal Cancer Have More Advanced Cancer Stage at Time of Diagnosis: A Community-Based Safety-Net Hospital Experience. J Community Health 2017;42:724-9. [Crossref] [PubMed]
- Arshad HM, Tetangco E, Shah N, et al. Racial Disparities in Colorectal Carcinoma Incidence, Severity and Survival Times Over 10 Years: A Retrospective Single Center Study. J Clin Med Res 2016;8:777-86. [Crossref] [PubMed]
- Damle RN, Flahive JM, Davids JS, et al. Examination of Racial Disparities in the Receipt of Minimally Invasive Surgery Among a National Cohort of Adult Patients Undergoing Colorectal Surgery. Dis Colon Rectum 2016;59:1055-62. [Crossref] [PubMed]
- Treska V, Fichtl J, Bruha J, et al. Liver Resections for Colorectal Metastases in Patients Aged Over 75 Years. Anticancer Res 2017;37:1529-33. [Crossref] [PubMed]
- Angelsen JH, Horn A, Sorbye H, et al. Population-based study on resection rates and survival in patients with colorectal liver metastasis in Norway. Br J Surg 2017;104:580-9. [Crossref] [PubMed]
- Mokdad AH, Dwyer-Lindgren L, Fitzmaurice C, et al. Trends and Patterns of Disparities in Cancer Mortality Among US Counties, 1980-2014. JAMA 2017;317:388-406. [Crossref] [PubMed]
- Warren JL, Klabune CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002;40:IV-3-18. [Crossref] [PubMed]
- Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol 2007;17:584-90. [Crossref] [PubMed]
- Ouellette JR, Small DG, Termuhlen PM. Evaluation of Charlson-Age Comorbidity Index as predictor of morbidity and mortality in patients with colorectal carcinoma. J Gastrointest Surg 2004;8:1061-7. [Crossref] [PubMed]
- Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol 2000;53:1258-67. [Crossref] [PubMed]
- Joinpoint Regression Program in Statistical Methodology and Applications Branch 2017, National Cancer Institute: Surveillance Research Program. Available online: https://surveillance.cancer.gov/joinpoint/, accessed February 1, 2017.
- Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med 2000;19:335-51. [Crossref] [PubMed]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [Crossref] [PubMed]
- SEER Cancer Stat Facts in Cancer Statistics. Surveillance, Epidemiology and End Results Program 2017, National Cancer Institute. Available online: https://seer.cancer.gov/statfacts/, accessed February 1, 2017.
- Vogelaar I, Ballegooijen M, Schrag D, et al. How much can current interventions reduce colorectal cancer mortality in the U.S.? Mortality projections for scenarios of risk-factor modification, screening, and treatment. Cancer 2006;107:1624-33. [Crossref] [PubMed]
- Itzkowitz SH, Winawer SJ, Krauskopf M, et al. New York Citywide Colon Cancer Control Coalition: A public health effort to increase colon cancer screening and address health disparities. Cancer 2016;122:269-77. [Crossref] [PubMed]
- Edwards BK, Noone AM, Mariotto AB, et al. Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 2014;120:1290-314. [Crossref] [PubMed]
- de Haas RJ, Wicherts DA, Andreani P, et al. Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann Surg 2011;253:1069-79. [Crossref] [PubMed]
- de Santibañes E, Fernandez D, Vaccaro C, et al. Short-term and long-term outcomes after simultaneous resection of colorectal malignancies and synchronous liver metastases. World J Surg 2010;34:2133-40. [Crossref] [PubMed]
- Shah SA, Haddad R, Al-Sukhni W, et al. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg 2006;202:468-75. [Crossref] [PubMed]
- van der Pool AE, Damhuis RA, Ijzermans JN, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis 2012;14:56-61. [Crossref] [PubMed]
- van der Geest LG, Lam-Boer J, Koopman M, et al. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis 2015;32:457-65. [Crossref] [PubMed]
- Parau A, Todor N, Vlad L. Determinants of survival after liver resection for metastatic colorectal carcinoma. J BUON 2015;20:68-77. [PubMed]
- Ito H, Are C, Gonen M, et al. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg 2008;247:994-1002. [Crossref] [PubMed]
- Schulman KA, Berlin JA, Harless W, et al. The effect of race and sex on physicians' recommendations for cardiac catheterization. N Engl J Med 1999;340:618-26. [Crossref] [PubMed]
- Werner RM, Asch DA, Polsky D. Racial profiling: the unintended consequences of coronary artery bypass graft report cards. Circulation 2005;111:1257-63. [Crossref] [PubMed]
- Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med 2005;41:23-9. [Crossref] [PubMed]
- Powe BD. Fatalism among elderly African Americans. Effects on colorectal cancer screening. Cancer Nurs 1995;18:385-92. [Crossref] [PubMed]
- Arnold LD, McGilvray MM, Kyle Cooper J, et al. Inadequate Cancer Screening: Lack of Provider Continuity is a Greater Obstacle than Medical Mistrust. J Health Care Poor Underserved 2017;28:362-77. [Crossref] [PubMed]
- Morris AM, Rhoads KF, Stain SC, et al. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg 2010;211:105-13. [Crossref] [PubMed]


