Cachexia, and not obesity, prior to pancreatic cancer diagnosis worsens survival and is negated by chemotherapy
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the 4th leading cause of cancer deaths (1) and is projected to become the second by 2020. Despite advances in the treatment and management of this malignancy, 5-year survival is still only 7%. Patients who have early stage disease are often those with the best outcomes (2,3). Therefore, increased attention has been paid to known risk factors for this disease including family history (4,5), diabetes (6,7) and obesity.
Obesity is a known risk factor for the development of PDAC (8-12). Obesity associated PDAC has been linked with decreased physical activity (8,13) and younger age of onset (14). Recent reports also suggest that obesity has a negative impact on outcomes in patients with a known diagnosis of PDAC (15).
Divergently, advanced pancreatic adenocarcinoma is better characterized by progressive weight loss and nutritional deterioration (16). It is estimated that up to 80% of these patients present with cachexia, defined as greater than a 5% weight loss over 6 months (17). Cachexia is a complex metabolic disorder that involves features of anorexia, anemia, and loss of adipose and skeletal muscle mass. In PDAC patients, it has been associated with reduced physical function, lower response rates to chemotherapy and radiotherapy (18-20). In resectable PDAC, recent data suggests cachexia and low serum albumin have been independently associated with poor surgical outcomes and lower survival (21,22). However, the effect of cachexia at diagnosis on survival in PDAC remains unclear (23). Herein, we characterize the significance of cachexia at presentation on clinical outcomes in PDAC.
To characterize the relationship between baseline body mass index (BMI) class, cachexia at diagnosis as defined by an international consensus, and overall survival in patients with PDAC, we reviewed a cohort of patients diagnosed with PDAC at Kaiser Permanente Medical Center.
Methods
This study was approved by the Kaiser Permanente Southern California (KPSC) Internal Review Board. KPSC is an integrated health care system that serves a socioeconomically diverse patient population representative of Southern California.
This study was a retrospective evaluation of a prospectively collected internal cancer registry of all PDAC cases from 2006–2014. All variables were extracted from electronic medical records and included: age, sex, ethnicity, stage, BMI class, survival, Charlson morbidity index, and receipt of chemotherapy. Patients greater than 18 years of age and greater than 1 year of continuous membership were included in the study. We excluded patients with histolopathologies other than adenocarcinoma, recurrent PDAC and missing PDAC stage or incomplete clinical data as noted above.
We defined our baseline BMI/weights as the measured value at 6 months prior to PDAC diagnosis. Cancer cachexia was defined as weight loss greater than 5% over the 6 months of evaluation, or weight loss greater than 2% in individuals already showing depletion according to current body weight and height (BMI <20 kg/m2 or sarcopenia) (3).
Statistical analysis
Demographic and clinical characteristics were compared between cachectic and non-cachectic individuals using t-tests for continuous variables, chi-square tests for categorical variables and a log-rank test for survival time. The association between cachexia and tumor location (pancreas head vs. other locations) was evaluated using multivariate logistic regression.
We used multivariate Cox proportional hazards regression to assess the influence of cachexia on survival. To evaluate effect measure modification of this relationship, we performed additional analyses stratified by race, BMI class, stage, receipt of surgery and receipt of chemotherapy. We tested for heterogeneity by fitting models with an interaction term for cachexia and the modifying variable.
All models included gender, age, ethnicity, BMI class, PDAC stage, surgery, chemotherapy, and Charlson comorbidity index as covariates. Patients were censored at death, last follow-up, or study conclusion on April 30, 2015. We verified the proportional hazards assumption using Schoenfeld residuals. All analyses were conducted with SAS 9.3 (Cary, NC, USA).
Results
Baseline characteristics
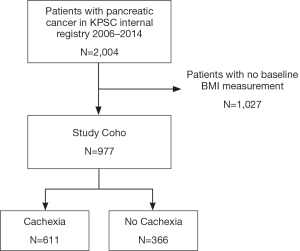
Our cohort consisted of 2004 patients diagnosed with PDAC from 2006–2014. Of these patients, 1,027 were excluded, as they did not have a baseline BMI (height and weight) measurement. There were no substantial differences in demographic or clinical characteristics among individuals with and without baseline BMI values. Of the remaining 977 patients, 611 (63%) were identified with cachexia while 366 (37%) did not meet this definition (Figure 1).
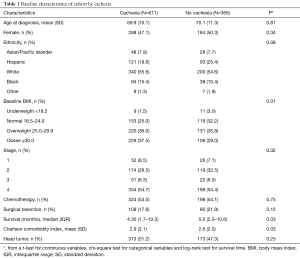
Baseline characteristics between cachectic and non-cachectic patients were evaluated and found to be similar. In our cohort, the mean age, gender ratio, and ethnicity between these two groups of patients were not statistically different (Table 1). However, there was a trend towards higher rates of cachexia in African American patients and lower rates in Hispanic patients. Similar percentages of patients in both groups received chemotherapy; 53% (cachexia) vs. 54% (non-cachexia). The most commonly used agents were gemcitabine (53%), 5-fluorouracil (13%), oxaliplatin (8%) nab-paclitaxel (6%), and irinotecan (5%).
Full table
Cachexia in PDAC patients was prevalent across all stages of disease. Specifically, 67% of stage 1 patients (n=78) and 63% of stage 4 patients (n=533) were found to have lost greater than 5% of their weight over the 6 months prior to diagnosis. Cachexia was also prevalent across all BMI classes. For example, of 20 patients with BMI <18.5 (underweight), 9 (45%) met the definition for cachexia. There was an association with elevated BMI class and increased incidence of cachexia (Table 1).
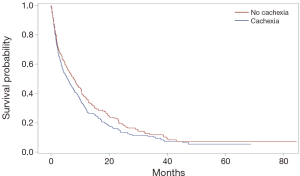
Cachexia in newly diagnosed PDAC was not associated with stage of diagnosis, surgical resection, or receipt of chemotherapy. However, there was an association with survival. Patients with cachexia had lower survival (median 4.3 months, IQR 1.7–10.0) compared to those without cachexia (median 5.2 months, IQR 2.0–10.6), log-rank P=0.03 (Figure 2). There was also an association between cachexia and an increased Charlson comorbidity index (P=0.03).
Multivariate analysis
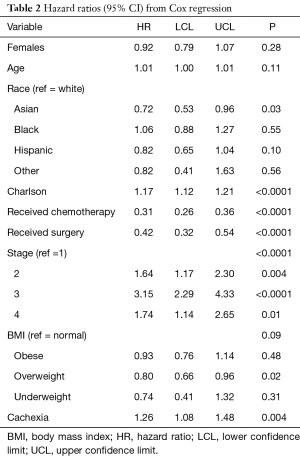
We observed no relationship between cachexia and tumor location in our multivariate logistic regression (OR 1.22, CI: 0.92–1.61, P=0.17). In the multivariate Cox regression, cachexia was independently associated with decreased overall survival (HR 1.24, CI: 1.06–1.45, P=0.01) after adjusting for gender, age, ethnicity, BMI class, PDAC stage, surgery, chemotherapy, and Charlson comorbidity index (Table 2). Notably, pre-diagnostic BMI class did not significantly affect survival outcomes. Gender, age, and ethnicity were also not associated with survival. Stage of disease, surgical resection, chemotherapy, and Charlson comorbidity index were associated with survival outcomes.
Full table
We detected no effect measure modification by race (Pinteraction=0.07), BMI class (Pinteraction=0.52), stage (Pinteraction=0.07), or receipt of surgery (Pinteraction=0.41). However, the effect of cachexia on survival outcomes was modified by receipt of chemotherapy. Cachectic patients who did not receive chemotherapy had a 40% increase in risk of death compared to non-cachectic patients (HR 1.40, CI: 1.12–1.75), while those receiving chemotherapy were unaffected by cachexia (HR 1.04, CI: 0.82–1.32, Pinteraction=0.01) (Figure 3).
Discussion
In the largest retrospective cohort of PDAC patients with weight loss evaluated to date, we found that 63% of newly diagnosed patients presented with the clinical diagnosis of cachexia. We report for the first time that cachexia at the time of diagnosis, defined as weight loss greater than 5% over 6 months, is an independent risk factor for worse survival in PDAC. This finding is independent of stage, surgical resection and BMI class. Previous reports in resected patients using lowest quartile of total psoas area (24) and BMI less than 18 (25) have demonstrated a similar association. In contrast to previous reports, we did not find any association between obesity or BMI class at diagnosis and survival (15,26). As BMI class was not associated with survival, further evaluation of weight loss by BMI subclass was not performed (27).
The prevalence of PDAC cachexia was unexpectedly high for patients at diagnosis. Approximately 2/3 of all patients were affected regardless of disease stage. Early and late stages were similarly affected. Cancer cachexia is often presumed to be associated with advanced disease and progress throughout the disease course (28). Theoretically, increasing tumor burden could lead to increased protein and muscle catabolism, however our data does not support this convention (29). Longitudinal studies in cancer cachexia assessing tumor burden will be needed to help better understand this relationship. Provocatively, our findings suggest that this clinical entity can be an early clinical finding and can actually herald the diagnosis of this lethal malignancy.
On multivariate analysis, we found the association between cachexia and survival was abrogated in those patients receiving chemotherapy. In fact, cachectic patients receiving systemic therapy did not have a worse survival than their non-cachectic counterparts. This suggests a therapeutic relationship between systemic chemotherapy and this clinical entity. To date, there have been no effective therapies for weight loss in PDAC patients (30). Weight gain was one of 3 clinical endpoints to assess clinical benefit in a randomized study of gemcitabine and 5-fluorouracil (31). In the gemcitabine arm, 24% had improvement in a composite endpoint of pain, performance status, and weight gain as compared to 5% in the 5-fluorouracil arm. Multi-agent systemic chemotherapy, our current standard of care, is perhaps even more effective in ameliorating weight loss and improving quality of life (32).
There is evidence that anorexia and hyper catabolism in cachexia are driven by cytokines, circulating hormones, neuropeptides, neurotransmitters, and tumor-derived factors (30). The improvement in outcomes in the cachectic patients receiving systemic therapy supports this understanding. Our data does not support exocrine pancreatic insufficiency and duodenal obstruction as plausible explanations for these results. They occur frequently in patients with pancreatic head lesions and associated biliary obstruction and frequently require nutritional support (33). In our cohort, there were no differences in those with pancreatic head vs. tail lesions.
Unfortunately, our study was not designed to assess weight change after diagnosis. However, we suspect that continued weight loss would impact survival negatively. Previous reports in resected, and locally advanced PDAC patients, have demonstrated that continued weight loss is an independent predictor for worse survival (21,34,35). In advanced patients, weight stabilization has been associated with improved survival and quality of life (36).
Cachexia in cancer patients has been associated with poor physical performance and this is a possible confounding factor. Performance status has been shown to be a prognostic factor in randomized control trials of advanced pancreatic cancer patients receiving chemotherapy (37). Our analysis preceded the diagnosis of pancreatic cancer and the study was not designed to ascertain KPS and ECOG prior to diagnosis. Other possible confounders including advanced stage and age were not significantly different in our cachexia and non-cachexia cohort.
Although obesity was not prognostic of survival outcomes, we did find an association between obesity and cachexia. In our cohort, a BMI of greater than 30 was associated with a higher prevalence of cachexia (68% vs. 32%, P<0.01). Sarcopenia obesity syndrome has been previously described and reports have suggested that cachexia is a common finding in overweight and obese patients with advanced gastrointestinal cancers (38). Our findings suggest that this association is independent of clinical stage and not simply explained by obese patients presenting later in their disease course. Perhaps this reflects a biologic characteristic of the underlying illness and physiologic host state.
To our knowledge, we report the largest retrospective experience assessing the influence of cachexia on clinical outcomes in PDAC. Cachexia in PDAC is more prevalent and impactful than previously perceived. Its effects on survival are across all stages of this disease and its impact is negated by receipt of chemotherapy. These findings suggest that weight loss in PDAC should be further investigated as a predictive and prognostic marker and it should be considered a covariate for stratification in randomized clinical trials. In addition, it’s prevalence in early stages of disease suggests an opportunity to use weight loss as screening tool for early detection.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board of Kaiser Permanente Medical Center (IRB protocol #10603) and informed consent was not required per policy.
References
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- Ariyama J, Suyama M, Satoh K, et al. Imaging of small pancreatic ductal adenocarcinoma. Pancreas 1998;16:396-401. [Crossref] [PubMed]
- Carpelan-Holmström M, Nordling S, Pukkala E, et al. Does anyone survive pancreatic ductal adenocarcinoma? A nationwide study re-evaluating the data of the Finnish Cancer Registry. Gut 2005;54:385-7. [Crossref] [PubMed]
- Schenk M, Schwartz AG, O'Neal E, et al. Familial risk of pancreatic cancer. J Natl Cancer Inst 2001;93:640-4. [Crossref] [PubMed]
- Silverman DT, Schiffman M, Everhart J, et al. Diabetes mellitus, other medical conditions and familial history of cancer as risk factors for pancreatic cancer. Br J Cancer 1999;80:1830-7. [Crossref] [PubMed]
- Batabyal P, Vander Hoorn S, Christophi C, et al. Association of diabetes mellitus and pancreatic adenocarcinoma: a meta-analysis of 88 studies. Ann Surg Oncol 2014;21:2453-62. [Crossref] [PubMed]
- Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 2005;294:2872-8. [Crossref] [PubMed]
- Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001;286:921-9. [Crossref] [PubMed]
- Coughlin SS, Calle EE, Patel AV, et al. Predictors of pancreatic cancer mortality among a large cohort of United States adults. Cancer Causes Control 2000;11:915-23. [Crossref] [PubMed]
- Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010;170:791-802. [Crossref] [PubMed]
- Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer 2007;120:1993-8. [Crossref] [PubMed]
- Aune D, Greenwood DC, Chan DS, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol 2012;23:843-52. [Crossref] [PubMed]
- Luo J, Margolis KL, Adami HO, et al. Obesity and risk of pancreatic cancer among postmenopausal women: the Women's Health Initiative (United States). Br J Cancer 2008;99:527-31. [Crossref] [PubMed]
- Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009;301:2553-62. [Crossref] [PubMed]
- Yuan C, Bao Y, Wu C, et al. Prediagnostic body mass index and pancreatic cancer survival. J Clin Oncol 2013;31:4229-34. [Crossref] [PubMed]
- Splinter TA. Cachexia and cancer: a clinician's view. Ann Oncol 1992;3:25-7. [Crossref] [PubMed]
- Ryan DP, Grossbard ML. Pancreatic Cancer: Local Success and Distant Failure. Oncologist 1998;3:178-88. [PubMed]
- Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491-7. [Crossref] [PubMed]
- Moses AG, Maingay J, Sangster K, et al. Pro-inflammatory cytokine release by peripheral blood mononuclear cells from patients with advanced pancreatic cancer: relationship to acute phase response and survival. Oncol Rep 2009;21:1091-5. [PubMed]
- Bachmann J, Ketterer K, Marsch C, et al. Pancreatic cancer related cachexia: influence on metabolism and correlation to weight loss and pulmonary function. BMC cancer 2009;9:255. [Crossref] [PubMed]
- Hendifar A, Osipov A, Khanuja J, et al. Influence of body mass index and albumin on perioperative morbidity and clinical outcomes in resected pancreatic adenocarcinoma. PLoS One 2016;11:e0152172. [Crossref] [PubMed]
- Bachmann J, Heiligensetzer M, Krakowski-Roosen H, et al. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J Gastrointest Surg 2008;12:1193-201. [Crossref] [PubMed]
- Ozola Zalite I, Zykus R, Francisco Gonzalez M, et al. Influence of cachexia and sarcopenia on survival in pancreatic ductal adenocarcinoma: a systematic review. Pancreatology 2015;15:19-24. [Crossref] [PubMed]
- Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic adenocarcinoma. J Gastrointest Surg 2012;16:1478-86. [Crossref] [PubMed]
- Pausch T, Hartwig W, Hinz U, et al. Cachexia but not obesity worsens the postoperative outcome after pancreatoduodenectomy in pancreatic cancer. Surgery 2012;152:S81-8. [Crossref] [PubMed]
- McWilliams RR, Matsumoto ME, Burch PA, et al. Obesity adversely affects survival in pancreatic cancer patients. Cancer 2010;116:5054-62. [Crossref] [PubMed]
- Martin L, Senesse P, Gioulbasanis I, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol 2015;33:90-9. [Crossref] [PubMed]
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- De Lerma Barbaro A. The complex liaison between cachexia and tumor burden Oncol Rep 2015;34:1635-49. (Review). [Crossref] [PubMed]
- Tan CR, Yaffee PM, Jamil LH, et al. Pancreatic cancer cachexia: a review of mechanisms and therapeutics. Front Physiol 2014;5:88. [Crossref] [PubMed]
- Burris HA 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [Crossref] [PubMed]
- Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23-9. [Crossref] [PubMed]
- Bruno MJ, Haverkort EB, Tijssen GP, et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut 1998;42:92-6. [Crossref] [PubMed]
- Hashimoto D, Chikamoto A, Ohmuraya M, et al. Impact of postoperative weight loss on survival after resection for pancreatic cancer. JPEN J Parenter Enteral Nutr 2015;39:598-603. [Crossref] [PubMed]
- Dalal S, Hui D, Bidaut L, et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Symptom Manage 2012;44:181-91. [Crossref] [PubMed]
- Davidson W, Ash S, Capra S, et al. Weight stabilisation is associated with improved survival duration and quality of life in unresectable pancreatic cancer. Clin Nutr 2004;23:239-47. [Crossref] [PubMed]
- Boeck S, Hinke A, Wilkowski R, et al. Importance of performance status for treatment outcome in advanced pancreatic cancer. World J Gastroenterol 2007;13:224-7. [Crossref] [PubMed]
- Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol 2013;31:1539-47. [Crossref] [PubMed]



