Age-related rates of colorectal cancer and the factors associated with overall survival
Introduction
In the US, the incidence of early onset colorectal cancer (CRC) for people under the age of 50 years has been increasing (1). This trend is alarming, with a statement highlighting its importance added to the most recent version of the National Comprehensive Cancer Network (NCCN) guidelines for colon and rectal cancer (2,3). Several causes for this increase have been described. From a biological perspective, more aggressive pathologic, molecular or genetic features have been identified which may be contributing to the increased incidence (4-7). Changes in risk factors among younger adults over time, including diets higher in fat and increasing obesity, are also in part, contributing to this rising trend (8,9).
In addition to these biologic etiologies, disparities in access to care for diagnosis and treatment of CRC among young adults has also been described. Several studies have characterized differences in the treatment of CRC, including access to surgery and chemotherapy based on age, race, geographic location and other socioeconomic factors like insurance status and income (10-15). Importantly, differences in access to these treatments have been associated with worse survival outcomes for patients with early-onset CRC of minority racial populations (16,17). The purposes of this study were to identify differences in both demographic and pathologic factors associated with the age-related rates of CRC and to assess the interaction these variables on overall survival (OS) in early onset CRC.
Methods
Patients
Jointly sponsored by the American Cancer Society and the American College of Surgeons, the National Cancer Data Base (NCDB) captures approximately 70% of the country’s cancer cases through its participating hospitals. A query of the NCDB 2006–2012 participant user files (PUFs) was performed to identify all patients with adenocarcinoma of the colon, rectosigmoid or rectum. At the time this study was performed, the NCDB provided data until 2013. Patients within the rectosigmoid and rectum PUFs were combined into a single group designated as rectum.
Patients with histology other than adenocarcinoma were excluded. For adenocarcinoma, the following International Statistical Classification of Diseases (ICD-O-3) codes were used: 8140–8148, 8200, 8260–8263, and 8480–8496. Patients were stratified by age (≤50 vs. ≥60 years), with ages 51–59 intentionally omitted from the analysis to minimize overlapping trends between these two age groups.
Patient factors reported in the NCDB include gender, race, ethnicity, income, education, insurance status, treatment facility type, geographic setting and Charlson-Deyo comorbidity score as a measure of comorbid conditions. Income is reported by the NCDB as the median household income based on zip code derived from the 2000 US Census. Similarly, level of education is captured in the NCDB as the proportion of residents in a patient’s zip code who did not graduate high school, which is divided this into four categories based on the proportion who did not graduate high school: lowest (≥29%), low (20–28.9%), high (14.1–19.9%), and highest (≤14%). The Charlson-Deyo comorbidity score is based on a number of reported ICD-9-CM secondary diagnosis codes and is reported as a single cumulative summary score as 0, 1, or ≥2.
Pathologic variables included site of tumor as it pertains to colon cancer (right, transverse, left, overlapping or not specified), grade, clinical and pathological TNM stage, serum carcinoembryonic antigen (CEA) level, lymphovascular invasion (LVI), and perineural invasion (PNI). Site of tumor was not applicable for patients with rectal cancer. For the purposes of this study, CEA level was analyzed by normal or elevated. LVI and PNI were also analyzed as dichotomous variables. The inclusion LVI status as a variable in the NCDB began 2010, and therefore the analysis was limited to these years (2010–2013) for LVI.
As this study used a national de-identified database, this was deemed exempt from our institutional review board.
Statistical analysis
Patient characteristics were reported by age group (≤50 vs. ≥60 years) using means, medians and standard deviations for continuous variables; and frequencies and relative frequencies for categorical data. Comparisons were made using the Wilcoxon rank sum and Pearson Chi-Square tests for continuous and categorical variables, respectively. The Holm-Bonferroni method was used to control the family-wise error rate within each analysis cohort.
OS was summarized by age group (≤50 vs. ≥60 years) using standard Kaplan-Meier methods and adjusted for Charlson-Deyo comorbidity status, where estimates of median and 3-/5-year OS were obtained with 95% confidence intervals (CI). Within each age group, the association between patient characteristics and OS were examined using Cox regression models. The models were fit using Firth’s method and hazard ratios (HRs) were obtained from model estimates. In order to identify any age effect on these associations, OS was then modeled as a function of each patient characteristic, age, and their interaction using a multivariable stratified Cox regression model after adjusting for the Charlson-Deyo comorbidity score. The test about the interaction term evaluated whether the HRs associated with the given patient characteristics differ between the two age groups. Separate models were fit for each patient characteristic. For each model a test about the interaction terms was conducted, which evaluated whether the HRs associated with the given patient characteristic differed between the two age groups. All models were adjusted by the Charlson-Deyo comorbidity status (stratification factor). The HRs with corresponding 95% CI were obtained from model estimates using standard methods. All model assumptions and fit were assessed graphically using Schoenfeld and Cox-Snell residual plots.
All analyses were conducted in SAS v9.4 (Cary, NC, USA) at a nominal significance level of 0.05.
Results
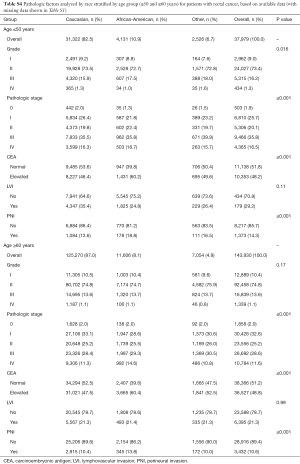
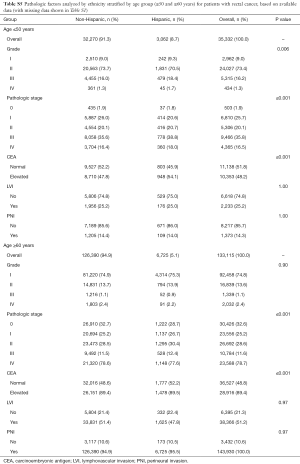
A total of 670,030 patients met the inclusion criteria as described above. Of these, there were 488,121 patients with colon cancer and 181,909 patients with rectal/rectosigmoid cancer. Table 1 shows the patient demographic and tumor characteristics for the colon cohort, and Table 2 shows data for the rectum cohort stratified by age group (≤50 and ≥60 years). Because of the large sample size, nearly all of these variables reached statistical significance (unless otherwise specified). As to be expected for both colon and rectum, patients ≥60 years had higher Charlson-Deyo comorbidity scores and were more likely to have Medicare for insurance. Non-White races comprised a higher proportion of patients in the ≤50 years group. Patients ≤50 years had lower levels of education in the colon group. Regarding pathologic variables, patients ≤50 years had higher overall rates of stage III/IV disease, more LVI, and more PNI compared to patients ≥60 years.
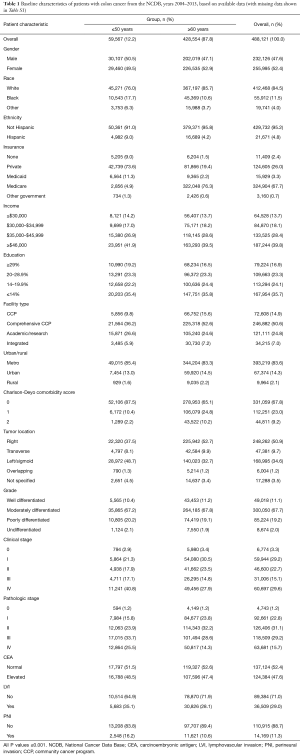
Full table
Full table
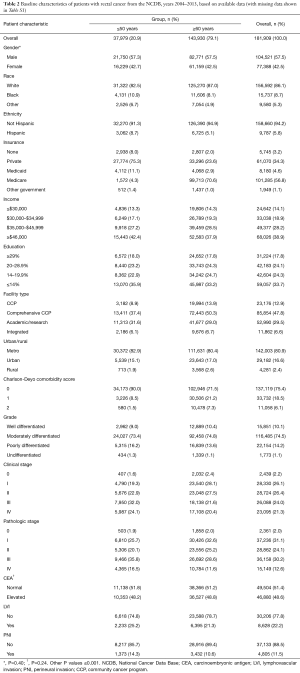
Full table
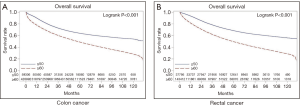
The Kaplan-Meier curves for OS are shown in Figure 1. The median follow-up was similar for patients with colon cancer and rectal cancer (62.4 and 62.1 months respectively). Overall, patients ≤50 years had superior outcomes to patients ≥60 years. For colon, the median OS for patients ≤50 years was not reached (NR) (95% CI: 127.5 months–NR) as compared to 57.5 months (95% CI: 57.1–57.9) for patients ≥60 years; and for rectum, the median OS was also not reached for ≤50 group compared to 59.1 months (95% CI: 58.5–59.7) for ≥60 group.
The multivariable analysis of patient demographic and tumor-related variables on OS for colon cancer when stratified by age is shown in Table 3. Analyses were adjusted for the Charlson-Deyo comorbidity scores. The ≤50 and ≥60 groups HRs correspond to the variable for the respective age groups. The ≤50 and ≥60 P values correspond to the overall effect of the variable on OS for the respective age groups. The interaction column contains two P values: (I) the first P value (corresponding to the row where the HRs are 1.00) is an overall test of whether the effect of the patient characteristic on OS differs between age groups; (II) the remaining P values compare the specific HRs between age groups.
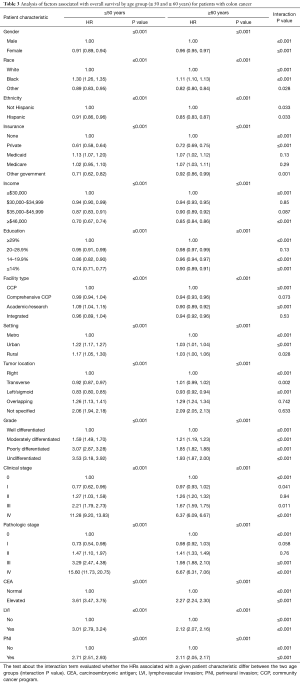
Full table
Most of the patient characteristics were significantly associated with OS, which in part may be due to the large sample size. Most strikingly, the association between many of these characteristics and OS differs between the age groups (i.e., have significant P values under the interaction column). The largest of these differences was observed for pathologic stage whereby stage IV disease had a significantly greater association on OS for patients ≤50 as compared to ≥60 (HR: 15.60 vs. 6.67, respectively). Other notable differences were found with respect to patients of Black/African American race and Hispanic ethnicity who demonstrated increasing tumor grade, elevated CEA, LVI and PNI. These interactions demonstrate that while having these factors is associated overall with poorer OS, the extent of the association was worse for patients ≤50.
The multivariable analysis of patient demographic and tumor-related variables on OS for rectal cancer when stratified by age is shown in Table 4. The largest of the differences was again observed for pathologic stage whereby stage IV disease had a significantly greater association on OS for patients ≤50 as compared to ≥60 (HR: 20.72 vs. 6.13, respectively). Other differences were found with respect to Black/African American race, Hispanic ethnicity, lower income level, increasing tumor grade, elevated CEA, LVI and PNI.
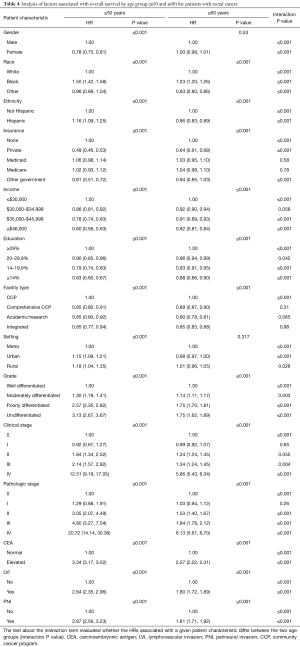
Full table
Tables S2-S5 show the distribution of pathologic factors by race or ethnicity stratified by age group for colon and rectum, respectively. The pathologic factors differed to a greater extent in the rectum group than in the colon group. For the colon group, the site of disease was also statistically significant with African Americans having a nearly 10% higher number of right sided colon cancer compared to Caucasians in the ≤50 group (Tables S2,S3). In contrast, the proportion of African Americans and Caucasians with right sided colon cancer in the ≥60 group was similar.
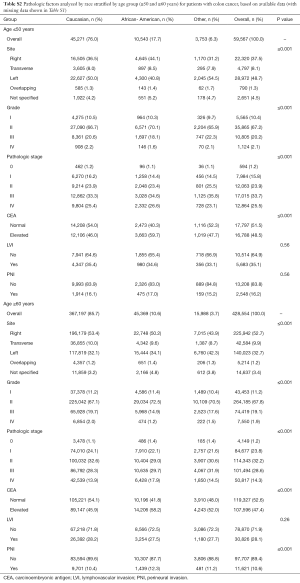
Full table
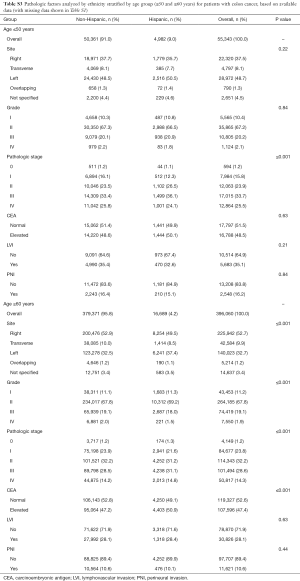
Full table
Full table
Full table
Discussion
This study is the first that uses a large nationwide database to investigate both demographic and pathologic factors as they pertain to early onset CRC and OS. The incidence of early onset CRC in the US has been increasing as noted through other databases such as the Surveillance, Epidemiology, and End Results Program (SEER) database or the North American Association of Central Cancer Registries (NAACCR) dataset (1-3,6,16,18,19). While these studies have focused on either the disparate demographic aspects or the pathologic features associated with early onset CRC, our study sought to look at both sets of characteristics and determine the association of these variables with OS of patients ≤50 and ≥60 years.
The current screening guidelines in the US recommend the age of 50 years to start screening the average risk individual and may therefore miss early onset CRC (20,21). In addition to issues related to the timing of CRC screening, behavioral factors are thought to contribute to the increased incidence of early onset CRC. Obesity and diet are associated with increased risk for CRC, and the rate of obesity among Americans has been reported to be as high as 34% (22). Physical inactivity, lack of exercise, tobacco use and consumption of alcohol are also related to increased CRC risk (23,24). These behavioral risk factors are more pervasive in populations with lower socioeconomic status in the US, thereby reflecting a relationship of demographic disparities and behavioral patterns that is associated with CRC (25,26).
Pathologic factors associated with increased early onset CRC have also been studied, and these factors may explain why CRC presents at advanced stages for younger patients as we and other have shown (27). These include tumor differentiation, LVI and PNI, which have been reported in other studies and were also included in our analysis (4,28). Signet-ring differentiation has been associated with poorer prognosis in patients with CRC (29,30). LVI and PNI have each been demonstrated to have prognostic significance in CRC (31-34). Our study provides the largest published cohort characterizing the association of both LVI and PNI on OS and supports its consideration in the prognostication of patients. Moreover, what is particularly novel with our study is the analysis of these factors as they relate to patients with early onset CRC.
Disparities in CRC care are becoming increasingly relevant in the US. For example, a recent study using the SEER database showed that OS for young patients with CRC was adversely associated with race whereby Black/African American and Hispanic patients had worse OS across all pathological stages as compared to White patients (16). Another study using the SEER database suggested that a reduction of racially-based treatment disparities may translate into improved survival for minorities (35). These findings are important as they may have implications on the guidelines published by the United States Preventive Services Task Force (USPSTF), which recommend CRC screening at age 50 (36). Other aspects of socioeconomic disparities, such as a lack of health insurance or being underinsured, have been shown to play a significant role in access to care including preventive services (37,38). These disparities may partly explain the increasing rates of early onset CRC, which often presents at an advanced stage as demonstrated by this and other studies (18,39,40). This study is the first to examine not only racial disparities but also other important socioeconomic disparities, including income and education level, that are related to early onset CRC and their association with OS.
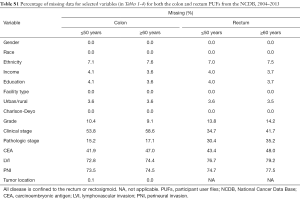
We acknowledge that there are limitations to the study. Regarding pathologic variables, LVI was included in the NCDB starting in 2010, and therefore the analysis with respect to LVI is more limited. In contrast, PNI was recorded in 2004. However, missing data and differences among contributing institutions with respect to pathologic analysis and interpretation are other limitations to the analysis. Table S1 lists the percentages of missing data within the colon and rectal PUF datasets. With respect to the disparities analysis, although the NCDB captures the majority of cancer cases in the US and allows for a robust statistical analysis, patients in minority populations or those with lower socioeconomic status may be less likely to be treated at the cancer centers participating in the NCDB. Therefore, the sample represented by the NCDB may be skewed toward White patients with higher socioeconomic status. While in general, the demographic variables included in the NCDB are quite comprehensive, the categories within each variable can be more limiting. For example, the NCDB uses the Charlson-Deyo comorbidity score as a measure of patient comorbidities, which is truncated to three values (zero indicates no comorbidities, one indicates a single selected comorbidity, and two indicate ≥1 of the selected comorbidities). Similarly, the NCDB has defined cutoff values for income and education derived from 2000 US Census data which are somewhat narrow and may be outdated in our study population.
Conclusions
In conclusion, this study reports new findings regarding both the pathologic and racial/socioeconomic disparities associated with age-related CRC and OS. Not only have younger patients presented at advanced stages (pathologic stage III/IV), they also had more aggressive biologic features including higher rates of CEA positivity, LVI and PNI. In addition, racial/socioeconomic disparities are also related to the increasing rate of early onset CRC and the poorer OS outcomes for minority patients. Interventions to address the disparities aspects of early onset CRC are highly needed. Earlier screening should be seriously considered in patients under 50 years who are African-American and Hispanic, as these populations present with more aggressive and advanced disease.
Acknowledgements
We thank the Commission on Cancer of the American College of Surgeons and American Cancer Society for access to the NCDB Participant User File.
Funding: This research was supported, in part, by the NCI Cancer Center Support Grant to Roswell Park Cancer Institute (CA016056) for the Biostatistics Shared Resource.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As this study used a national de-identified database, this was deemed exempt from the institutional review board.
References
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015;150:17-22. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Colon Cancer (Version 4.2014). Available online: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- National Comprehensive Cancer Network. Rectal Cancer (Version 4.2014). Available online: http://www.nccn.org/professionals/physician_gls/pdf/rectum.pdf
- Yantiss RK, Goodarzi M, Zhou XK, et al. Clinical, pathologic, and molecular features of early-onset colorectal carcinoma. Am J Surg Pathol 2009;33:572-82. [Crossref] [PubMed]
- Pilozzi E, Maresca C, Duranti E, et al. Left-sided early-onset vs late-onset colorectal carcinoma: histologic, clinical, and molecular differences. Am J Clin Pathol 2015;143:374-84. [Crossref] [PubMed]
- I Ben-Aharon RP, Elkabets M, Battaglin F, et al. Early onset colorectal cancer - does the difference lie in epigenetics? Vienna, Austria: European Cancer Congress 2015: abstract 179.
- Kaz AM, Wong CJ, Dzieciatkowski S, et al. Patterns of DNA methylation in the normal colon vary by anatomical location, gender, and age. Epigenetics 2014;9:492-502. [Crossref] [PubMed]
- Steins Bisschop CN, van Gils CH, Emaus MJ, et al. Weight change later in life and colon and rectal cancer risk in participants in the EPIC-PANACEA study. Am J Clin Nutr 2014;99:139-47. [Crossref] [PubMed]
- Karahalios A, English DR, Simpson JA. Weight change and risk of colorectal cancer: a systematic review and meta-analysis. Am J Epidemiol 2015;181:832-45. [Crossref] [PubMed]
- Alnasser M, Schneider EB, Gearhart SL, et al. National disparities in laparoscopic colorectal procedures for colon cancer. Surg Endosc 2014;28:49-57. [Crossref] [PubMed]
- Ayanian JZ, Zaslavsky AM, Fuchs CS, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol 2003;21:1293-300. [Crossref] [PubMed]
- Hao Y, Landrine H, Jemal A, et al. Race, neighbourhood characteristics and disparities in chemotherapy for colorectal cancer. J Epidemiol Community Health 2011;65:211-7. [Crossref] [PubMed]
- Gabriel E, Thirunavukarasu P, Al-Sukhni E, et al. National disparities in minimally invasive surgery for rectal cancer. Surg Endosc 2016;30:1060-7. [Crossref] [PubMed]
- Gabriel E, Al-Sukhni E, Nurkin S. Commentary on "Insurance Status, Not Race, is Associated With Use of Minimally Invasive Surgical Approach for Rectal Cancer". Ann Surg 2018;267:e29-e30. [Crossref] [PubMed]
- Gabriel E, Thirunavukarasu P, Al-Sukhni E, et al. National Disparities in Surgical Approach to T1 Rectal Cancer and Impact on Outcomes. Am Surg 2016;82:1080-91.
- Holowatyj AN, Ruterbusch JJ, Rozek LS, et al. Racial/Ethnic Disparities in Survival Among Patients With Young-Onset Colorectal Cancer. J Clin Oncol 2016;34:2148-56. [Crossref] [PubMed]
- Andaya AA, Enewold L, Zahm SH, et al. Race and colon cancer survival in an equal-access health care system. Cancer Epidemiol Biomarkers Prev 2013;22:1030-6. [Crossref] [PubMed]
- Rahman R, Schmaltz C, Jackson CS, et al. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the United States. Cancer Med 2015;4:1863-70. [Crossref] [PubMed]
- Teng A, Lee DY, Cai J, et al. Patterns and outcomes of colorectal cancer in adolescents and young adults. J Surg Res 2016;205:19-27. [Crossref] [PubMed]
- Carethers JM. Screening for colorectal cancer in African Americans: determinants and rationale for an earlier age to commence screening. Dig Dis Sci 2015;60:711-21. [Crossref] [PubMed]
- Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology 2003;124:544-60. [Crossref] [PubMed]
- Ogden CL, Carroll MD, Kit BK, et al. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 2014;311:806-14. [Crossref] [PubMed]
- Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer 1996;73:1134-40. [Crossref] [PubMed]
- Lee SE, Jo HB, Kwack WG, et al. Characteristics of and risk factors for colorectal neoplasms in young adults in a screening population. World J Gastroenterol 2016;22:2981-92. [Crossref] [PubMed]
- Doubeni CA, Major JM, Laiyemo AO, et al. Contribution of behavioral risk factors and obesity to socioeconomic differences in colorectal cancer incidence. J Natl Cancer Inst 2012;104:1353-62. [Crossref] [PubMed]
- Doubeni CA, Laiyemo AO, Major JM, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study. Cancer 2012;118:3636-44. [Crossref] [PubMed]
- Abdelsattar ZM, Wong SL, Regenbogen SE, et al. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer 2016;122:929-34. [Crossref] [PubMed]
- Chang DT, Pai RK, Rybicki LA, et al. Clinicopathologic and molecular features of sporadic early-onset colorectal adenocarcinoma: an adenocarcinoma with frequent signet ring cell differentiation, rectal and sigmoid involvement, and adverse morphologic features. Mod Pathol 2012;25:1128-39. [Crossref] [PubMed]
- Benedix F, Kuester D, Meyer F, et al. Influence of mucinous and signet-ring cell differentiation on epidemiological, histological, molecular biological features, and outcome in patients with colorectal carcinoma. Zentralbl Chir 2013;138:427-33. [PubMed]
- Inamura K, Yamauchi M, Nishihara R, et al. Prognostic significance and molecular features of signet-ring cell and mucinous components in colorectal carcinoma. Ann Surg Oncol 2015;22:1226-35. [Crossref] [PubMed]
- Lim SB, Yu CS, Jang SJ, et al. Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis Colon Rectum 2010;53:377-84. [Crossref] [PubMed]
- Shirouzu K, Isomoto H, Kakegawa T. Prognostic evaluation of perineural invasion in rectal cancer. Am J Surg 1993;165:233-7. [Crossref] [PubMed]
- Betge J, Pollheimer MJ, Lindtner RA, et al. Intramural and extramural vascular invasion in colorectal cancer: prognostic significance and quality of pathology reporting. Cancer 2012;118:628-38. [Crossref] [PubMed]
- Poeschl EM, Pollheimer MJ, Kornprat P, et al. Perineural invasion: correlation with aggressive phenotype and independent prognostic variable in both colon and rectum cancer. J Clin Oncol 2010;28:e358-60; author reply e361-2.
- Valeri L, Chen JT, Garcia-Albeniz X, et al. The Role of Stage at Diagnosis in Colorectal Cancer Black-White Survival Disparities: A Counterfactual Causal Inference Approach. Cancer Epidemiol Biomarkers Prev 2016;25:83-9. [Crossref] [PubMed]
- U.S. Preventive Services Task Force. Screening for colorectal cancer: recommendation and rationale. Ann Intern Med 2002;137:129-31. [Crossref] [PubMed]
- Abdus S, Mistry KB, Selden TM. Racial and Ethnic Disparities in Services and the Patient Protection and Affordable Care Act. Am J Public Health 2015;105 Suppl 5:S668-75. [Crossref] [PubMed]
- Hargraves JL, Hadley J. The contribution of insurance coverage and community resources to reducing racial/ethnic disparities in access to care. Health Serv Res 2003;38:809-29. [Crossref] [PubMed]
- Mojica CM, Glenn BA, Chang C, et al. The Relationship between Neighborhood Immigrant Composition, Limited English Proficiency, and Late-Stage Colorectal Cancer Diagnosis in California. Biomed Res Int 2015;2015:460181. [Crossref] [PubMed]
- Reyes-Ortiz CA, Eschbach K, Zhang DD, et al. Neighborhood composition and cancer among Hispanics: tumor stage and size at time of diagnosis. Cancer Epidemiol Biomarkers Prev 2008;17:2931-6. [Crossref] [PubMed]


