Fluoropyrimidine-induced cardiac toxicity: challenging the current paradigm
Introduction
Fluoropyrimidine chemotherapy [5-fluorouracil (5-FU) and the prodrug capecitabine] are the cornerstone drugs in the treatment of gastrointestinal (GI), breast, and other solid malignancies (1). Their use and effectiveness has been limited by hematologic and systemic toxicity. Among the latter, a spectrum of cardiac toxicity occurs, ranging from coronary ischemia, systolic left ventricular dysfunction, arrhythmias and sudden death with an incidence ranging from 1–18% (2). Higher risk may be associated with longer duration infusions, pre-existing coronary artery disease (CAD), pre-treatment structural heart disease, chronic kidney disease (CKD), concurrent cisplatin chemotherapy and prior chest radiation (3-7). In spite of these risk factors, most cardiac toxicity occurs in patients without concurrent chemotherapy or structural heart disease. Aside from the acute cardiovascular risk, fluoropyrimidine cardiac toxicity has resulted in early treatment termination with the potential for under treatment of the cancer and negative impact on survival.
We summarize the natural history and clinical presentation of fluoropyrimidine cardiotoxicity with its proposed mechanism and a review of historical management strategies. Further, we describe our single-center experience with chest pain/acute coronary syndrome (ACS) associated with fluoropyrimidine use and recommendations for rechallenge after fluoropyrimidine-induced chest pain in the largest case series to date.
Mechanism and clinical presentation
Although fluoropyrimidine cardiotoxicity is a well-known phenomenon, there is still debate regarding mechanism of action of the drug, variations in clinical presentation of the cardiotoxicity, and the ability to identify the high-risk patient. The most accepted mechanism for fluoropyrimidine-induced cardiac toxicity is coronary vasospasm leading to ischemia (2,8-11). Moreover, the most common cardiac symptom is chest pain with or without transient electrocardiogram (ECG) changes (2,12). Silent ischemia has also been reported (ECG changes without chest pain) and continuous ECG monitoring studies suggest that up to 2/3 of patients may have silent ischemia during therapy (13,14). Even though there is speculation that pre-existing CAD and traditional cardiac risk factors may predict a high-risk patient population (14), to date, there is no useful or accepted clinical prediction tool to guide therapy.
5-FU is administered intravenously either as a bolus as part of the 5-fluorouracil, folinic acid, and oxaliplatin (FLOX) regimen or variable duration continuous infusion (24–96 hours) as part of the folinic acid, fluorouracil and oxaliplatin (FOLFOX) regimen. The development of symptoms varies according to delivery mode. Bolus infusion 5-FU is typically administered between 2–15 minutes. Chest pain usually occurs during the “push” or immediately after the first cycle. In the absence of symptoms during cycle 1, it is unusual to occur subsequently. Chest pain description suggests a cardiac origin and typically is associated with ST segment elevation on the ECG suggestive of acute ST segment elevation myocardial infarction (“initial bolus pattern”). This is the classic textbook description of 5-FU large epicardial coronary artery spasm.
Less well described is the chest pain associated with continuous infusion 5-FU. Chest pain associated with continuous infusion may also occur with the first or second chemotherapy cycle, typically between 24–72 hours after infusion initiation. The pain may be atypical compared to classical angina, i.e., occurring at rest, resolving spontaneously. Symptoms may also recur cyclically during the infusion and persist following infusion completion. Many patients find these symptoms tolerable and complete the planned infusion course. Because of the intermittent nature and variable tolerability of the symptoms, it is not unusual for patients to initially ignore and not report this chest pain. Additionally, since infusions are administered in the outpatient setting without telemetry, accompanying ECG changes, if present, are generally not captured. If treatment is continued, during subsequent cycles, symptoms predictably recur progressively earlier and are more intense with longer duration (“continuous exposure pattern”). The characteristics of the bolus and infusion patterns are listed in Table 1. Similarly, oral capecitabine is administered daily and the metabolism is similar to continuous infusional 5-FU. As such, the development of chemotherapy associated chest pain is similar to the continuous infusion pattern.

Full table
In most patients, including those with acute recurrent of injury patterns on the ECG, regardless of the culprit fluoropyrimidine, coronary arteriography fails to reveal evidence of epicardial CAD (15).
Management strategies—historical review
There is current agreement that the acute management of patients with fluoropyrimidine-associated chest pain, when recognized, is to stop the drug and administer short acting sublingual and/or long-acting nitrates and/or calcium channel blockers. However, there are little consensus data about pretreatment prophylaxis. In a seminal study in 1990, Eskilsson et al. described 58 patients randomized to either pre-treatment or no treatment with oral verapamil (120 mg three times a day) (16). All patients received a combination of cisplatin and infusional 5-FU and there was no statistically significant difference between groups in ischemia prevention (12% vs. 13%) (16). Salepci et al. found no benefit to pre-treatment with angiotensin converting enzyme inhibitors (ACE-inhibitors) (17). The results of these two studies discouraged major subsequent investigation of pretreatment prophylaxis. Similarly, there is no current established role for pre-treatment exercise testing, coronary computed tomography angiogram (CTA) or cardiac MRI nor for the routine use of continuous ECG monitoring or routine measurement of biomarkers (troponin, NT-proBNP) during treatment.
It is also widely accepted that rechallenge after documented cardiac toxicity without a change in original drug dosing is associated with recurrence cardiotoxicity rates as high as 80% and reported death rates as high as 18% (7). This led Sorrentino and colleagues in 2012 to comment that “reintroducing 5-FU to patients with a history of cardiotoxicity following prior 5-FU administration is not currently advised” (7).
Little has changed in the past 5 years. Possible alternative treatment options that have been reported include:
- Discontinuation of the fluoropyrimidine with or without a switch to a “second” line non-fluoropyrimidine regimen (18-20) or switch from 5-FU to oral capecitabine (21);
- Dose reduction by 20–50% dose and retreat with the original drug (6,22);
- Switch from infusional (FOLFOX) to bolus 5-FU (FLOX) (23). This is mechanistically logical since toxicity may be more likely related to accumulated metabolites rather than peak dose (24,25);
- For all approaches, the addition of some combination of nitrates and calcium channel blockers has been a consistent part of every rechallenge protocol (6,26-28).
Some physicians base their rechallenge decision on intent of chemotherapy. With palliative intent, there has been a tendency to not rechallenge in contrast to a higher likelihood of rechallenge with curative intent.
Methods and results
Management strategies—our institutional approach to fluoropyrimidine chest pain
Step 1: acute treatment
The acute management of patients with fluoropyrimidine associated chest pain when recognized is to discontinue the drug. If the patient is in an acute care setting, short acting sublingual and/or long-acting nitrates and/or calcium channel blockers are acutely administered. If the patient is at home and reports chest pain, the culprit drug is stopped and the patient is instructed to go to the nearest emergency room.
Step 2: initial assessment
Acute assessment for myocardial damage includes history, ECG, cardiac troponin, and echocardiography. These parameters define classification into a high or low risk pattern of fluoropyrimidine-induced cardiotoxicity. High risk pattern patients are defined by any combination of ongoing chest pain, dynamic ECG changes, troponin elevation or new wall motion abnormalities on echocardiogram. Conversely, low risk pattern patients have none of these changes. Bolus administration of 5-FU is more likely to lead to a high-risk pattern while patients receiving continuous infusional 5-FU or oral capecitabine more often fall into the low risk category.
Step 3: subsequent assessment according to risk pattern
Unless there is a major contraindication, all high-risk pattern patients presenting with anginal symptoms should undergo emergent coronary arteriography. Obstructive CAD in the territory suggested by the ECG is revascularized and patients are subsequently treated with standard dual antiplatelet therapy. Patients with no obstructive CAD on angiography are monitored for 1–3 days post angiography depending on symptom resolution—the latter is dependent on total clearance of drug which is non-linear and highly variable (29).
Low risk pattern patients “incidentally” recognized after “cycle” completion and symptom resolution are not hospitalized. However, we routinely assess these symptom-free patients for underlying CAD with either exercise testing or coronary CTA.
Step 4: rechallenge—institutional cases series
We report a single institution series of 11 consecutive patients with fluoropyrimidine-induced chest pain who have been rechallenged with the culprit drug. Patient characteristics are listed in Table 2. Eight patients were initially treated with continuous 5-FU (FOLFOX) and three patients were treated with capecitabine. Two of the three capecitabine patients were successfully rechallenged and completed planned chemotherapy. Nine patients had GI adenocarcinoma and two patients had metastatic breast cancer. There were five males and six females with age ranging from 48–74 years at the start of fluoropyrimidine therapy. Three of 11 patients had baseline hypertension (controlled with ACE-I and diuretic), two also had hyperlipidemia (treated with a statin), and one patient also had non-insulin dependent type 2 diabetes. No other patient had pre-treatment cardiac risk factors. No patient was actively smoking or using tobacco products and all had calculated glomerular filtration rate (GFR) >60 mL/min. No patient had any prior history of CAD or structural heart disease.
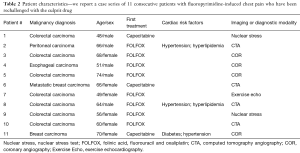
Full table
Every patient described chest pressure or pain at rest without an exertional component. Patients #3–5 presented to the emergency department (ED) during their first cycle of FOLFOX with persistent chest pain and ST segment elevation on ECG consistent with ACS. Patient #11 presented to the ED with chest pain and ST segment elevation on day 6 of capecitabine cycle 1. All 4 patients had emergent coronary arteriography. The other 7 patients (5 FOLFOX and 2 capecitabine) initially ascribed their symptoms to heartburn that was intermittent and recurrent during treatment. They all tolerated recurrent episodes of short-duration, low intensity chest discomfort and reported symptoms only subsequent to the completion of the treatment cycle: 5 after cycle 2 and 1 after cycle 3. By the time of their evaluation, they were all pain free and had no ST or T wave changes on the ECG.
Patients #3–5 and 11 had coronary arteriography and of these, patient #5 had a stent placed in the RCA while patients #3, 4 and 11 had no demonstrable epicardial CAD. Three patients had coronary CTA scans with no evidence of CAD or coronary calcification. Three patients had exercise nuclear stress studies and 1 patient had stress echocardiography, both without inducible ischemia.
The first 4 FOLFOX patients #2–5 in our series were hospitalized for FOLFOX rechallenge and pre-treated with long-acting nitrates (isosorbide dinitrate), two calcium blockers (long acting nifedipine and short acting diltiazem). A cardiologist was physically present for the initiation of the 5-FU infusion and immediately available on-site for the entire infusion. The patients all had continuous ECG monitoring. All had recurrent chest pain promptly relieved with sublingual nitroglycerine (SL NTG): one with associated ST elevation; four with no associated ECG changes. In all four, the timing of symptom onset occurred several hours earlier than the prior cycle. For all four, the infusion was interrupted for 60 minutes and restarted after all cardiac medications were uptitrated proportional to blood pressure tolerance. In all, chest pain predictably recurred within a shorter duration of the most recent rechallenge. For patients #2, 4, 5, we stopped treatment after the second rechallenge recurrence. Patient #3 required IV nitroglycerin that only enabled infusion continuation for 3 hours before chest pain recurred and infusional 5-FU was then discontinued.
A similar experience of chest pain recurrence with infusional 5-FU rechallenge was reported in a single patient. A 47-year-old male with adenocarcinoma of the colon and no demonstrable CAD had 5-FU-induced vasospasm. Rechallenge was accomplished over 10 cycles in spite of the fact that the patient experienced chest pain radiating to the shoulders and jaw with each cycle in spite of continuous IV NTG and IV morphine. He required an average of 10 SL NTGs [0–16] per 5-FU infusion (30).
As a result of our early experience and this case report, we recognized that patients with coronary ischemia associated with continuous infusional 5-FU are unlikely to achieve safe rechallenge without recurrent ischemia. We abandoned any further rechallenge with continuous infusion 5-FU after treatment-induced chest pain thought to be due to coronary spasm.
After discussion with our GI medical oncology team, there was agreement to switch the cancer therapy from FOLFOX to non-inferior FLOX regimen. In the latter, bolus 5-FU is substituted for continuous infusion. This regimen is less convenient for the patient with a more frequent treatment schedule but it avoids prolonged 5-FU continuous infusion. Our rechallenge treatment flow is detailed in Table 3.
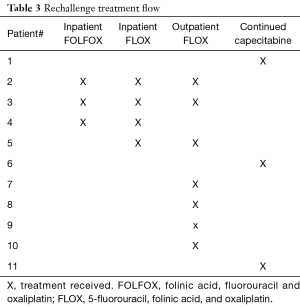
Full table
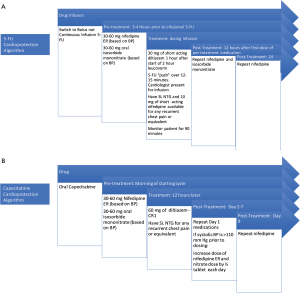
Subsequently three of these initial four patients #2, 4, 5 described above were re-admitted to the hospital for the first FLOX cycle. We describe our pre-treatment protocol for 5-FU rechallenge in Figure 1A. Bolus infusion was tolerated without any recurrent chest pain, ECG changes or detectable troponin release. We monitored them in the hospital with telemetry for 24 hours post infusion without any cardiac event. Patient #3’s oncologist considered the completed chemotherapy adequate for adjuvant therapy and no further chemotherapy was administered.
As a result of successful in-patient experience, we subsequently shifted our protocol to the outpatient setting for all subsequent cycles for all of the patients in our series.
Outpatient 5-FU treatment
All eight of these patients tolerated bolus 5-FU given over a 15-minute period. Patients were initially monitored in the chemotherapy infusion suite for 4 hours post bolus injection. We subsequently reduced this time period to 90 minutes without any adverse effect. We routinely re-dosed anti-spasm medication at home the evening of treatment (long-acting nifedipine and nitrate) and morning after with long-acting nifedipine. With this pre/post treatment regimen, all patients were able to complete multiple FLOX cycles without recurrent chest pain or complication. One patient with metastatic peritoneal carcinomatosis has had >20 months of weekly 5-FU without any recurrent angina and with stable metastatic disease. For all FLOX patients, a cardiologist has been present for every bolus infusion.
Outpatient capecitabine treatment
Capecitabine is typically administered orally twice daily for 1 week followed by a week off and the pattern is repeated. The oral dosing during the week of therapy is similar to continuous infusion of 5-FU. We describe the rechallenge algorithm for capecitabine administration with the use of anti-anginals in Figure 1B.
The three capecitabine patients were treated with the same prophylactic regimen without reduction of the original capecitabine dose. For all, we measured blood pressure daily throughout treatment week and prophylactically up-titrated each drug daily and with each succeeding cycle to achieve the maximally tolerated and effective doses of calcium blockers and nitrates. The patient with colorectal cancer (patient #1) completed planned capecitabine chemotherapy without recurrent chest pain and the breast cancer patient (patient #6) was able to complete 6 months of capecitabine without recurrent angina until progression of disease led to an alternative non-fluoropyridimine regimen. The third capecitabine patient #11 was only able to complete 6 days of rechallenge in spite of ultimate 50% capecitabine dose reduction coupled with blood pressure limited “triple therapy” due to recurrence of chest pain that was consistently relieved with SL NTG (Table 2).
Clinical follow up
For all patients, there have been no cardiac events or evidence of recurrent coronary spasm after completion of therapy with discontinuation of prophylactic medications upon therapy completion. This is consistent with the absence of late recurrence of drug-induced spasm reported in the literature (31).
Discussion
The fluoropyrimidines continue to be the cornerstone drug in the treatment of GI and other solid tumor malignancies. Treatment induced chest pain may lead to discontinuation of effective and potentially curable chemotherapy. There is no universally accepted approach to rechallenge once cardiac chest pain is suspected and/or documented. Several alternative strategies have been reported that include chemotherapy without fluoropyrimidines, dose reduction, switching from infusional to bolus regimen and the addition of cardio-protective, anti-spasm medication. All prior reports have major limitations in size and approach. Most are non-randomized retrospective, often single patient reports or small series with incomplete data. There has been no consistency to treatment with various combinations of medications and dosing. All have shown inconsistent and mixed results except for the largest successful case series reported by Ambrosy et al. (32). They successfully completed capecitabine rechallenges in five patients with capecitabine-induced chest pain after pretreatment with oral diltiazem.
We report the largest successful case series of rechallenge with 5-FU after suspected and/or documented 5-FU coronary artery spasm. Like prior reports, our series is also non-randomized and the diagnosis of coronary spasm was inferred in patients based on clinical presentation, evaluation and response to therapy. Only tree had documented ECG changes and had emergent coronary arteriography; one had underlying CAD and two had no evidence of obstructive CAD and were presumed to have coronary artery spasm. Nevertheless, this is an important contribution that suggests a treatment algorithm with 100% success in safe 5-FU rechallenge and 66% success rate for capecitabine.
A latent period from treatment initiation to symptom emergence has been similar for both 5-FU and capecitabine. It has been postulated previously that this time lag is related to a slow metabolizer phenotype and that it takes some finite time dependent on the rate of metabolism to reach a “spasm threshold.” This explains the predictable time course and symptom escalation over time. During bolus infusion, these metabolites are less likely to accumulate to the spasm threshold and patients remain spasm-free. We have screened for DDYP (dihydropyrimadine dehydrogenase) polymorphism in patient #6 (capecitabine) and patient #2 (5-FU), and neither had a mutation. We initially postulated that if patients were identified as “slow metabolizers” we could simply manipulate dosing by reducing the fluoropyridimine dose by 50% to reduce the likelihood for spasm. A research test is commercially available for DDYP measurements and we thought it might be useful, not for screening, but for treatment decisions after suspected fluoropyrimidine-induced chest pain was suspected. Because of the excessive cost, long turn-around time for results and unproven efficacy, we have not incorporated this test into our algorithm.
Our data do not provide a risk score to predict fluoropyrimidine-induced chest pain. There was no age-related risk and only 3 of 11 patients had traditional cardiac risk factors in our small series. One patient was found to have underlying CAD after treatment initiation. A larger prospective series with multivariate analysis is needed to define a high-risk patient that might benefit from pre-treatment cardioprotection.
In addition to the treatment algorithm detailed in Figure 1, we offer several the following recommendations prior to the and during treatment with fluoropyrimidine based chemotherapy:
- Every patient should have a thorough cardiac history documented and a baseline pretreatment ECG to be used mainly for comparison if cardiac toxicity is suspected during treatment;
- Every patient should be instructed to immediately report any chest discomfort or pain or perceived shortness of breath associated with their treatment;
- Given that there may be an elevated risk of ischemia in those with pre-existing CAD (14), we recommend cautious use and proactive patient education about manifestations of ischemia during 5-FU and capecitabine treatment in potentially high-risk patients with pre-treatment maximization of anti-anginal regimens. The presence of stable CAD should not be an absolute contraindication to appropriate oncologic use of these drugs with decisions and use made on a case by case basis;
- Patients presenting as ACS generally have ST segment elevation and present with the first bolus exposure to 5-FU. The absence of an ACS-like presentation is characterized by recurrent symptoms that may be atypical (at rest), that progressively occur earlier after each cycle initiation with increased frequency and severity during continued treatment. The ECG at evaluation almost never shows ST segment elevation or ischemic T wave changes. Nevertheless, fluoropyridimine-induced coronary spasm should be suspected in the latter patients;
- We do not withhold rechallenge of fluoropyridimine chemotherapies based on “intent”: effective palliative 5-FU containing regimens can extend progression free status (PFS) when 5-FU is effective in stabilizing metastatic disease. We believe that if the regimen is effective from an oncologic standpoint, we pursue “safe” rechallenge;
- We also offer rechallenge under closely supervised conditions in higher risk patients who have had evidence of treatment-related myocardial infarction (ECG changes and or troponin elevation) or those with documented coronary spasm and obstructive CAD that is subsequently revascularized;
- For both high and low risk patients, this presents an opportunity for primary and/or secondary prevention around diet, exercise, tobacco-free behavior and aggressive lipid management.
Limitations
Although we have had 100% success in 5-FU rechallenge without any major adverse cardiac event or recurrent spasm, we recognize that this is a small consecutive series from a single institution. No treated patient had a pretreatment history of CAD or structural heart disease, CKD or prior therapeutic radiation exposure, and so our results may not be transferable to patients with one or more of these additional risk factors. None of our patients had a ST-segment elevation myocardial infarction (STEMI) as a result of coronary spasm. Although there are scattered and isolated case reports of the safety of rechallenge in patients with treatment-induced myocardial infarction, there may not be enough evidence to dispel the caution of rechallenge reported in the literature to extend our practice of rechallenge to other centers. In spite of time-concentrated doses of 2 calcium channel blockers and long-acting oral nitrate therapy, we did not encounter any symptomatic hypotension and the only treatment-related side effect was self-limited headache managed with aspirin or acetaminophen.
We also add a word of caution—our protocol did not include any patients who had any treatment-induced acute left ventricular systolic dysfunction. The pathophysiology of this presentation is not due to coronary artery spasm. Possible explanations include a variant of stress cardiomyopathy or a hypersensitivity reaction to the fluoropyrimidine leading to acute fulminant myocarditis. We would not extrapolate our protocol to the latter clinical scenario.
Conclusions
Fluoropyrimidine-induced chest pain is a real entity in clinical practice. Successful rechallenge can be safely and effectively accomplished in the out-patient setting with careful cardiac monitoring and the combined use of anti-spasm calcium channel blockers and long-acting nitrates. We believe that this is an important contribution since the fluoropyrimidines are widely used treatment anchors for multiple solid tumors and if cardiac toxicity is common (4–5% of all treated patients), the ability to safely continue potentially curative regimen helps solve a large problem in oncology and cardio-oncology. We are in the process of establishing an international database to validate the safety and efficacy of this rechallenge protocol.
Acknowledgements
None.
Footnote
Conflicts of Interest: The abstract was submitted to the American Heart Association Conference and has been accepted for poster presentation (conference in November 2017).
Ethical Statement: This manuscript did not meet criteria for a formal IRB process as the cases were done under clinical care and quality improvement of our existing treatment processes.
References
- Network NCC. NCCN guidelines for treatment of cancer by site. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site
- Polk A, Vaage-Nilsen M, Vistisen K, et al. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: a systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 2013;39:974-84. [Crossref] [PubMed]
- Wacker A, Lersch C, Scherpinski U, et al. High incidence of angina pectoris in patients treated with 5-fluorouracil. A planned surveillance study with 102 patients. Oncology 2003;65:108-12. [Crossref] [PubMed]
- Meydan N, Kundak I, Yavuzsen T, et al. Cardiotoxicity of de Gramont's regimen: incidence, clinical characteristics and long-term follow-up. Jpn J Clin Oncol 2005;35:265-70. [Crossref] [PubMed]
- Kosmas C, Kallistratos MS, Kopterides P, et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: a prospective study. J Cancer Res Clin Oncol 2008;134:75-82. [Crossref] [PubMed]
- Jensen SA, Sorensen JB. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol 2006;58:487-93. [Crossref] [PubMed]
- Sorrentino MF, Kim J, Foderaro AE, et al. 5-fluorouracil induced cardiotoxicity: review of the literature. Cardiol J 2012;19:453-8. [Crossref] [PubMed]
- Tsavaris N, Kosmas C, Vadiaka M, et al. Cardiotoxicity following different doses and schedules of 5-fluorouracil administration for malignancy -- a survey of 427 patients. Med Sci Monit 2002;8:PI51-7. [PubMed]
- Jensen SA, Sorensen JB. 5-fluorouracil-based therapy induces endovascular injury having potential significance to development of clinically overt cardiotoxicity. Cancer Chemother Pharmacol 2012;69:57-64. [Crossref] [PubMed]
- Polk A, Vistisen K, Vaage-Nilsen M, et al. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol Toxicol 2014;15:47. [Crossref] [PubMed]
- Sudhoff T, Enderle MD, Pahlke M, et al. 5-Fluorouracil induces arterial vasocontractions. Ann Oncol 2004;15:661-4. [Crossref] [PubMed]
- Alter P, Herzum M, Soufi M, et al. Cardiotoxicity of 5-fluorouracil. Cardiovasc Hematol Agents Med Chem 2006;4:1-5. [Crossref] [PubMed]
- Rezkalla S, Kloner RA, Ensley J, et al. Continuous ambulatory ECG monitoring during fluorouracil therapy: A prospective study. J Clin Oncol 1989;7:509-14. [Crossref] [PubMed]
- Meyer CC, Calis KA, Burke LB, et al. Symptomatic cardiotoxicity associated with 5-fluorouracil. Pharmacotherapy 1997;17:729-36. [PubMed]
- Cardinale D, Colombo A, Colombo N. Acute coronary syndrome induced by oral capecitabine. Can J Cardiol 2006;22:251-3. [Crossref] [PubMed]
- Eskilsson J, Albertsson M. Failure of preventing 5-fluorouracil cardiotoxicity by prophylactic treatment with verapamil. Acta Oncologica 1990;29:1001-3. [Crossref] [PubMed]
- Salepci T, Seker M, Uyarel H, et al. 5-Fluorouracil induces arterial vasoconstrictions but does not increase angiotensin II levels. Med Oncol 2010;27:416-20. [Crossref] [PubMed]
- Layoun ME, Wickramasinghe CD, Peralta MV, et al. Fluoropyrimidine-induced cardiotoxicity: manifestations, mechanisms, and management. Curr Oncol Rep 2016;18:35. [Crossref] [PubMed]
- Gradishar W, Vokes E, Schilsky R, et al. Vascular events in patients receiving high-dose infusional 5-fluorouracil-based chemotherapy: the University of Chicago experience. Med Pediatr Oncol 1991;19:8-15. [Crossref] [PubMed]
- Labianca R, Luporini G. 5-fluorouracil cardiotoxicity: the risk of rechallenge. Ann Oncol 1991;2:383. [Crossref] [PubMed]
- Abdelrahman M, McCarthy MT, Yusof H, et al. Successful capecitabine rechallenge following 5-fluorouracil-induced Takotsubo syndrome. Oxf Med Case Reports 2016;2016:47-50.
- Saif MW, Shah MM, Shah AR. Fluoropyrimidine-associated cardiotoxicity: revisited. Expert Opin Drug Saf 2009;8:191-202. [Crossref] [PubMed]
- Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. [Crossref] [PubMed]
- Shaib W, Lee V, Saif MW. Bolus 5-Fluorouracil as an alternative modality to infusion 5-Fluorouracil in a patient with rectal cancer and capecitabine-induced cardiotoxicity. In Vivo 2009;23:821-6. [PubMed]
- Cerny J, Hassan A, Smith C, et al. Coronary vasospasm with myocardial stunning in a patient with colon cancer receiving adjuvant chemotherapy with FOLFOX regimen. Clin Colorectal Cancer 2009;8:55-8. [Crossref] [PubMed]
- Rateesh S, Luis SA, Luis CR, et al. Myocardial infarction secondary to 5-fluorouracil: not an absolute contraindication to rechallenge? Int J Cardiol 2014;172:e331-3. [Crossref] [PubMed]
- Deboever G, Hiltrop N, Cool M, et al. Alternative treatment options in colorectal cancer patients with 5-fluorouracil- or capecitabine-induced cardiotoxicity. Clin Colorectal Cancer 2013;12:8-14. [Crossref] [PubMed]
- Cianci G, Morelli MF, Cannita K, et al. Prophylactic options in patients with 5-fluorouracil-associated cardiotoxicity. Br J Cancer 2003;88:1507-9. [Crossref] [PubMed]
- McDermott BJ, van den Berg HW, Murphy RF. Nonlinear pharmacokinetics for the elimination of 5-fluorouracil after intravenous administration in cancer patients. Cancer Chemother Pharmacol 1982;9:173-8. [Crossref] [PubMed]
- Vargo CA, Blazer M, Reardon J, et al. Successful completion of adjuvant chemotherapy in a patient with colon cancer experiencing 5-fluorouracil-induced cardiac vasospasm. Clin Colorectal Cancer 2016;15:e61-3. [Crossref] [PubMed]
- Becker K, Erckenbrecht JF, Haussinger D, et al. Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs 1999;57:475-84. [Crossref] [PubMed]
- Ambrosy AP, Kunz PL, Fisher GA, et al. Capecitabine-induced chest pain relieved by diltiazem. Am J Cardiol 2012;110:1623-6. [Crossref] [PubMed]