Management of locally advanced rectal cancer in the elderly: a critical review and algorithm
Introduction
In 2016, the estimated number of new cases of rectal cancer was 39,220 in the United States. Although the incidence and death rates of colorectal cancer declined by 3% per year from 2003 to 2012, colorectal cancer remains the second leading cause of death in men ages 60–79 and the third leading cause of death in men over 80 years old and in women over 60 years old (1).
Among patients with colorectal cancer, rectal cancer accounts for about 30% of cases. This high mortality rate highlights the need for improved awareness of possible issues in caring for an elderly group of patients (2). Management of rectal cancer is challenging and involves multidisciplinary care. The data to guide treatment of elderly patients with rectal cancer are sparse since the elderly population has been underrepresented in prospective clinical trials involving colorectal cancer (3).
Older patients diagnosed with colorectal cancer are less likely to be referred to medical oncology, to receive standard of care chemotherapy (4) and more likely to undergo dose reductions and early termination of therapy (5). A population-based study in rectal cancer showed that age was the strongest determinant of treatment and that with advancing age there was a decline in the proportion of patients receiving standard of care adjuvant therapy even after adjusting for co-morbidities (6).
The goal of this consensus statement is to review the available literature and establish a treatment algorithm to aid the oncologist in treatment planning and decision-making in the elderly population with rectal adenocarcinoma. For the purposes of this article, we employ the definition of elderly as ≥70 years, but we recognize that the definition of the elderly can vary from 70 to 75 years, depending on the study.
Geriatric assessment tools and predictors
Chronological age is not an accurate tool in predicting treatment-related outcome and toxicities. The International Society of Geriatric Oncology (SIOG) has strongly recommended the use of a systematic comprehensive geriatric assessment (CGA) in elderly patients with cancer (7). Multiple factors are included in the CGA: functional status including activities of daily living (ADL) and instrumental ADL (IADL), nutritional status, co-morbidities, polypharmacy, cognitive function and psychosocial status (8-10) (Table 1). A multidimensional geriatric assessment (MGA) identified three different categories of patients based on their life expectancy; “fit patients” who may receive the same treatments as younger patients, “vulnerable patients” who require tailored treatment approaches and “frail patients” who are only candidates for supportive care (20) (Table 2). The CGA is time and labor consuming; therefore, it is seldom used in clinical practice. Another prognostic tool is the multidimensional prognostic index (MPI) that has been validated recently as a predictor of mortality and length of stay during hospitalization in elderly patients. The MPI generates a score between 0 and 1 and identifies the risk of mortality; a higher MPI is associated with a higher mortality risk (21,22). A modified cancer-specific MPI has been developed and found to be an accurate predictive tool for 1-year mortality in older cancer patients (23). When compared to the MGA, the MPI has a greater discriminatory power for 12-month mortality than the MGA in a prospective study of 160 patients ≥70 years old with locally advanced or metastatic solid cancer (24).
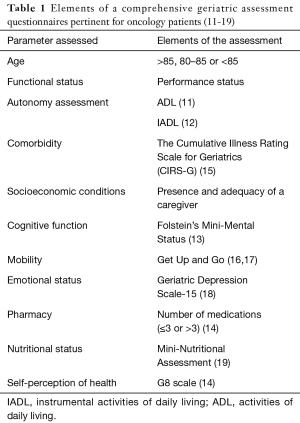
Full table
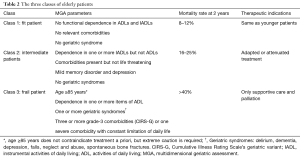
Full table
Two studies have evaluated the impact of a geriatric assessment on treatment-decisions (25,26) and found that a CGA did significantly influence the decision-making in 30 to 80% of patients. Consequently, a systematic review assessed the diagnostic performance of seven different screening methods of frailty to predict the presence of impairment on a CGA in elderly patients. These frailty screening methods had insufficient discriminative power to refine patient selection; therefore, the authors recommended for elderly patients to receive a CGA (27).
Studies have attempted to elucidate the role of chemotherapy and factors contributing to inadequate delivery of chemotherapy in this patient population. Baseline depression and instrumental dependencies were associated with functional decline in patients who are ≥70 years receiving first line chemotherapy (28). To further assess the risk factors associated with increased toxicity in older patients with cancer, a number of tools incorporating geriatric assessment are under evaluation. In a prospective multicenter study, 562 patients ≥70 years of age were assessed based on twenty-four parameters including laboratory studies, instrumental ADL, performance status, chemotherapy regimen toxicity, etc. The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score was found to be a useful predictive tool which could distinguish the risk of toxicity for this patient population (29). In another report, a predictive model for grade 3 to 5 toxicities was developed in a cohort of 500 patients aged ≥65 years who underwent comprehensive assessment that included socioeconomic setting, treatment modalities and geriatric assessment of function, comorbidities, cognition, activity level and social support. Patients were stratified in three groups based on the risk of chemotherapy toxicity: low, intermediate and high risk (30). The main criticism of this study was lack of an in-office performance mobility test that could potentially add significant value to the scoring system (31).
Though data from these series and population-based studies provide some insight into factors to consider while making treatment decisions, widespread application of geriatric assessment still remains to be adopted. The American Society of Oncology convened a sub-committee to address lack of evidence for treating elderly patients with cancer and after analyzing the current evidence; a set of recommendations was formulated to address this question, which are detailed in Table 3 (32).

Full table
Multiple dilemmas may exist in the decision-making for elderly patients with locally advanced rectal cancer, which center on underlying patient factors (comorbidities, functional status, geriatric assessments, and overall life expectancy) and the anticipated tolerance of the standard of care therapy (preoperative chemoradiation, subsequent surgery, and postoperative chemotherapy). Herein, we seek to evaluate the available evidence regarding treatment administration and provide an algorithm based on these data.
Neoadjuvant chemoradiation in elderly patients
Neoadjuvant long-course chemoradiation is the gold standard for locally advanced rectal cancer with no evidence of distant metastasis followed by surgical resection and adjuvant chemotherapy. Compared to post-operative chemoradiation, neoadjuvant chemoradiation was associated with improved local control and reduced toxicity (33). Combined modality therapy has been shown to decrease the risk of loco-regional recurrence (34-36).
Using the Surveillance, Epidemiology, and End Results (SEER) database, Chang et al. evaluated 21,390 patients with localized rectal cancer and found that with each 5-year increase in age ≥70 years, there was a 37% increase in the relative risk for cancer-related mortality and fewer cancer-directed surgeries, and less use of radiotherapy. The effect of age ≥70 years resulted in a 31% increase in cancer-specific mortality (37). Patients who were younger, had positive lymph nodes, comorbid condition and nonblack race were more likely to receive chemoradiation (38). Nevertheless, these data suggest possible under-treatment of the elderly.
An audit based on Swedish Rectal Cancer Registry was done to assess the impact of age on outcome in rectal cancer. A total of 15,104 patients with rectal cancer of which 42% were ≥75 years old were evaluated. Distant metastases were less frequently diagnosed in patients ≥75 years old, and patients were less likely to undergo preoperative radiotherapy and surgical resection. However, when surgery was performed, a Hartmann’s procedure was more frequently used. The authors concluded that age had an impact on treatment decisions, but they did not find a significant difference in survival at 5 years or local recurrence rate between patients <75 years of age and ≥75 years (39). It is possible that such studies are subject to selection bias in patients ≥75 years who may not have been treated. Likewise, a large multi-institutional Canadian study including more than 1,100 patients treated with neoadjuvant chemoradiation followed by curative intent surgery found similar disease-free survival (DFS), cancer-specific survival and overall survival in patients <70 years of age and ≥70 years (40). Choi et al. evaluated 160 patients who underwent neoadjuvant chemoradiation did not observe a difference in tolerance to chemotherapy, complete pathological response rate or treatment related complications in older patients compared to a younger cohort (41).
In a series of 56 patients with a mean age of 78 years, compliance rates for radiotherapy and chemotherapy were 91% and 41% respectively with adherence comparable to younger patients. The rate of grade gastrointestinal (GI) >3 toxicity was 14.3% (42). Another small series evaluated 36 patients >70 years with rectal cancer with a Cumulative Illness Rating Scale-Geriatric and categorized patients as “fit” and “vulnerable”. All patients were able to complete a full course of radiotherapy (50.4 Gy with bolus and continuous infusion 5-FU) and “vulnerable” patients did not experience higher acute toxicity compared to “fit” patients and were able to similarly tolerate concurrent therapy compared to “fit” patients (43). As a result of perceptions, rather than objective scales to determine fitness, there may be disparities in administration of combined modality therapy in older adults as demonstrated by a series of 267 patients treated at the Boston Medical Center and Boston Veteran’s Hospital. In this study, the odd of initiating therapy for patients >71 years was reduced by 22% after adjusting for comorbid status. In this study, only 56 of patients completed chemoradiation without dose reduction or delay, and completion of therapy was more likely with preoperative chemoradiation (44). A study from France also found disparities in the treatment of older patients (ages 80–84 years). Patients older than age 85 years were treated preferentially with primary chemoradiation and non-surgical management and demonstrated a 5-year overall survival rate of 45% and DFS rate of 65% (with the overall survival rate being inferior and the DFS being similar or improved compared to younger patients). The authors found that the differences in survival rates between elderly and younger patients were attributable to complications and co-morbidity, highlighting the need for a CGA (45). Disparities in care were also detected in a Canadian study that showed that age ≥75 year was the only factor impacting whether patients underwent surgery alone or surgery plus chemoradiation. Patients who received only surgery had a risk of death 2.35 times greater than elderly patients treated with trimodality therapy (46). Other series have also corroborated under-treatment of the elderly, which can be detrimental to quality of life due to recurrent disease (6,47).
A SEER analysis using data from 2004–2011 identified 4,121 elderly patients, >75 years, with locally advanced rectal cancer, which were divided into four groups: (I) surgery only (n=1,460); (II) radiation only (n=577); (III) neoadjuvant radiation therapy (RT) (n=1,498); (IV) adjuvant RT (n=586). The 5-year cancer-specific survival showed the best outcome for patients in the neoadjuvant RT group (70.4%), followed by adjuvant RT (60.4%), followed by surgery only (52.1%), and followed by RT only (27.7%) (48). A Mayo Clinic study of 160 elderly rectal cancer patients age ≥75 revealed that neoadjuvant chemoradiation did not provide a survival benefit for stage II (n=66) rectal cancer but did portend a benefit for stage III (n=94) rectal cancer compared to no neoadjuvant therapy (49). Another SEER study evaluating 2,886 stages II–III rectal cancer patients showed that completing postoperative chemoradiation resulted in a lower cancer-related mortality compared to their counterparts with no adjuvant therapy. Stage III patients were more likely to receive chemoradiation compared to stage II patients (50). The Mayo series may have been underpowered to evaluate the benefit of adjuvant chemoradiation in stage II patients.
Regarding the toxicities of neoadjuvant chemoradiation, in an unplanned subset analysis of the ACCORD12/PRODIGE2 phase III trial, preoperative chemoradiotherapy led to more severe grade 3/4 toxicities (25.6% vs. 15.8%, P=0.01) and more permanent stomas (33.3% vs. 22.8%, P=0.014) in elderly patients (≥70 years) who were less often operated on than younger patients (<70 years) (95.8% vs. 99.0%, P=0.008). The relative number of interventions per surgery type, treatment efficacy in terms of R0 resection rate, and complete pathological response (14.7% vs. 16.9%; P=0.55) were nearly identical between the two categories. Therefore, the treatment team must contemplate safe and individualized therapy for each patient (51).
Other series have identified comorbidity indices as a reasonable method to preemptively assess patient toxicity and outcomes in the elderly rectal cancer population being treated with chemoradiation (52,53). In contrast, multiple other series have advocated for trimodality management of locally advanced rectal cancer, citing similar outcomes to younger patients (54-58).
Short-course radiation
Short-course radiation has been shown to be an appropriate option for patients with stage III rectal cancer with effective local control and comparable overall survival to long course chemoradiation (59-61).
In a subgroup analysis of the Dutch total mesorectal excision (TME) study, which showed an improvement in overall survival with addition of short course radiation (5 Gy ×5 fractions) before surgery, patients >75 years of age did not gain a survival advantage, and the mortality rates during the first 6 months were higher compared to younger patients (62). Similarly, treatment related complications were significantly higher in 455 patients ≥70 years old treated with short course radiation or long course chemoradiation ± intraoperative radiotherapy. On multivariate analysis, age >70, comorbidity and having ≥ two complications were significantly associated with worse survival (63). A study from the Netherlands analyzed a total of 642 patients aged 75 years and older with stage pT2–T3, N0–2 rectal carcinoma treated with surgery alone (n=296) and pre-operative radiation followed by surgery alone (n=346). When compared to surgery alone, short-course preoperative radiotherapy resulted in a decreased rate of local recurrence in patients ≥75 years (6% vs. 2%). However, postoperative complications occurred more frequently in the irradiated group especially deep infections and local wound problems but the 30-day mortality rate was similar between the two groups. Severe comorbidity, chronic obstructive pulmonary disease (COPD), diabetes and cerebrovascular disease were associated with a 4-fold increase in the 30-day mortality. On multivariate analysis, postoperative complications predicted 5-year survival (64).
Short-course radiation is not uniformly suitable for elderly patients who are surgical candidates as available studies suggest increased complication rates and lack of a survival advantage. For those with severe comorbidities and poor performance status, it should be considered with caution since the side effects and post-operative complications are not trivial.
Brachytherapy
Brachytherapy has been used as primary preoperative therapy for T1 or T2/T3, node negative or small node positive rectal cancer, as a dose escalation boost and in the palliative setting.
Papillon popularized contact X-ray brachytherapy (CXB) 50 kVp in the 1950s–1960s in Europe. It has been widely used in the treatment of early stage rectal cancer as a definitive treatment or in the adjuvant setting after surgery with excellent outcomes (65-68). Local treatment with radical radiotherapy is an alternative to radical surgery in patients with T1N0M0 rectal cancer. Sun Myint has established selection criteria for suitability of radical contact radiotherapy; mobile, non-ulcerative, exophytic tumors <10 cm from the anal verge, tumor size <3 cm or occupying less than 1/3 of the rectal circumference, T1 well to moderately differentiated tumors with no lymphovascular invasion (69). In patients with T1/T2 rectal cancer treated with radical radiotherapy including contact radiotherapy, local failure and overall survival were 7–25% and 60–96%, respectively (66,69-74). Myint strongly encouraged the consideration of contact radiotherapy as an alternative to surgery in elderly patients and those with a high anesthetic risk with low early stage cancer (75).
Others reported their experience with the combination of CXB brachytherapy with external beam RT in patients with early stage and advanced T2–T3 rectal tumors and achieved good local control (60–70% at 5 years) (76-78).
Endoluminal high-dose rate (HDR) Ir-192 is gaining popularity in North America. In a study by Corner et al. HDR Ir-192 brachytherapy was used in 79 patients with locally advanced rectal tumors of whom 52 were unfit for surgery and were treated radically. In this study, the patient population was predominantly elderly with a median age was 82 years. Objective local tumor response was achieved in 85% of patients of whom 58% had a complete response and 27% had a partial response. The median survival of patients treated with a palliative intent was 6 months and 18.5 months for patients treated radically (79). In conclusion, contact radiotherapy or HDR brachytherapy are appropriate treatment options for early stage and locally advanced rectal tumors in patients unfit for surgery and in the elderly population.
Surgical management of rectal cancer in the elderly
The combined effects of an aging population and rectal cancer as an age-related disease will continue to increase the need for surgery in the elderly population (80,81). Surgical treatment of rectal cancer remains fundamental in the management of colorectal cancer. However, with multimodality treatment approaches for rectal cancer, alternative options and even non-surgical approaches become possible definitive treatments for older patients who are perceived to be at a higher surgical risk than younger patients. The risks for surgery alone in the elderly need assessment, independent of chemotherapy and radiation. Due to age and fragility, additional surgical options may be considered. Furthermore, in patients with multiple medical comorbidities, preoperative optimization and an interdisciplinary effort among anesthesiology, cardiology, and primary medical providers can help to better determine postoperative risks. Multiple tools also can be used to determine risk scores for complications and may help to inform surgical choices (82).
The first question therefore is whether age alone is a relative contraindication to surgical management of rectal cancer. Elderly patients make up an increasingly higher percentage of patients presenting with rectal cancer, but the percentage of patients in this age group undergoing curative surgery is lower and emergency surgery is more often the indication compared to younger patients (83,84). Studies evaluating age as an independent risk factor for complications have not been conclusive, with some studies finding an association with age, and others finding increased comorbidities without a specific effect on post-operative course (85-89). The SEER Database has been helpful in clarifying the impact of age on rectal cancer surgery. Chang et al. found that for patients over 70 years, there was a decrease in cancer related surgery with more local excision and less radical surgery across all stages of rectal cancer (37). A second SEER analysis compared survival in elderly patients based on operative vs. non-operative approaches. Elderly patients had improved cancer-specific survival as well as overall survival when treated with surgical management of their rectal cancers. The elderly population did show divergence in cancer-specific survival and overall survival suggesting that the mortality in the elderly was due to non-cancer related causes, supporting the use of radical therapy in the elderly (90). Operative approaches to rectal cancer are unique in the multiple options available for management of tumors. In general, earlier stage tumors can be addressed by either abdominal or transanal surgery with different risks and benefits to each.
Transanal surgery (local excision)
Transanal surgery in general offers the advantage of decreased operative morbidity, but a higher recurrence rate. Transanal endoscopic microsurgery (TEM), allows removal of rectal lesions through a 40-mm operating proctoscope with rectal insufflation (91). Advantages of the TEM system include improved visualization of tumors, decreased recurrence rates compared to standard transanal excision and access to more proximal tumors (92-95). Many studies have evaluated the efficacy of TEM in addressing rectal cancer (95-99). A meta-analysis evaluated 860 patients with early stage rectal cancer of which 303 underwent a TEM and 557 received standard surgical approaches including total mesorectal dissection. No significant difference was identified for DFS or distant metastatic disease. However, there was an increase in local recurrence with TEM compared to standard surgery (100). In general, TEM is best for low-risk cancers, well to moderately differentiated T1, without lymphovascular invasion that are less than 3–4 cm in size (101). Although there is limited data specific to the elderly, the relative increased safety of the TEM approach, faster operative time, decreased blood loss, shorter hospital-stay, and decrease in stoma formation make it an attractive approach to an elderly patient with more comorbidities (97).
A second area where local excision of a rectal tumor has been employed is in patients that have been treated with neoadjuvant chemoradiation as a means of evaluating for complete pathologic response or for resection for cure of the downstaged tumor. Two large prospective trials, the ACOGSOG Z6041 and the CARTS study have evaluated the role of local excision following chemoradiation therapy with promising results (102,103). There is currently no study of this technique specific to the elderly population.
TME
Current standard therapy for rectal surgery includes performing a TME for improved local control of disease and survival (104,105). However, Rutten et al. evaluated the impact of TME on the elderly and found that older patients did not have improved survival compared to younger patients and that the mortality within the 6-month period following treatment was significantly higher in patients over 75 years compared to a younger cohort (14% vs. 3.9%) (62). A subsequent evaluation by the same group again revealed no improvement with TME in the elderly population prompting them to suggest alternate methods of treatment for the elderly population (106).
Laparoscopic surgery
One area that has been evaluated specifically with respect to the elderly population is laparoscopic surgery. Laparoscopic approaches to rectal cancer have increased over the past 20 years with laparoscopy providing decreased narcotic intake, shorter hospital stays, faster return to activity compared to those undergoing open surgery (107-109). A number of studies have specifically looked at this comparison in the elderly population (110-113). Manceau et al. evaluated 446 consecutive patients grouping them into 10-year intervals from under 45 to older than 64 years. Elderly patients had a higher American Society of Anesthesiologists (ASA) score and higher Charlson comorbidity index preoperatively with higher cardiovascular, pulmonary and neurological comorbidities. Despite these differences, there was no difference in post-operative complications and age was not a significant independent risk factor for post-operative morbidity (111). Otsuka et al. evaluated short and long-term outcomes in patients undergoing laparoscopic surgery and compared octogenarians to case matched controls between the ages of 60–69. They similarly found that the ASA score was significantly higher in the octogenarian group but this did not correlate with increased post-operative complications and long-term cancer specific survival (91% in the octogenarian group and 95.7% in the case matched controls). There was an increased rate of permanent stoma in the elderly group from either abdominoperineal or Hartmann’s procedure compared to the middle-aged group (112). Recently, comparison between laparoscopic and open surgery has been evaluated in two large multicenter trials, ALACART and ACOSOG Z6051 with both showing that laparoscopic surgery did not meet non-inferiority for rectal cancer patients (114,115). These results should prompt further study and evaluation of the role of laparoscopic surgery in the management of rectal cancer.
Robotic surgery
Robotic surgery is an emerging technology in the surgical management of rectal cancer and is another option for a minimally invasive approach to rectal surgery. A number of small studies have evaluated the role of robotics in the treatment of rectal cancer (116-118) and the ROLAAR trial (NCT01736072) was established to compare the role of robotics in rectal cancer surgery. No published series exist regarding the elderly and robotic approaches to rectal cancer.
Stoma rates
Another significant difference in the elderly population compared to the younger population is the use of stomas as part of the surgical management (119). This is likely related to concern about fecal incontinence following low pelvic reconnection in the elderly population. One study out of the Netherlands did show significantly worse quality of life based on Wexner and fecal incontinence quality of life scores. However, in a number of other studies, elderly patients that do have a low pelvic anastomosis are generally satisfied with their control and functional results (120-122). In general, after a preoperative assessment of fecal continence, and a discussion of the potential risks of fecal urgency and soiling, elderly patients should be offered option of sphincter-sparing surgery for low rectal tumors.
While surgery remains integral in the management of the elderly patient with rectal cancer, multidisciplinary and patient specific factors need to be considered when developing the optimal treatment plan.
Adjuvant chemotherapy for the elderly rectal cancer patient
Specifically, with regards to the elderly patient, there is a paucity of data about the use of postoperative chemotherapy for rectal cancer patients treated with chemoradiation. In a study by Margalit et al. evaluating the tolerability of combined modality therapy for elderly rectal cancer patients (n=36, ages ≥75 years), only 39% of patients were able to complete ≥4 months of adjuvant chemotherapy (123). Lund et al. examined the comparative effectiveness of postoperative chemotherapy using the SEER Medicare database. Two groups of patients were compared in terms of mortality rates: (I) postoperative 5-FU or capecitabine to no treatment (n=666) and (II) postoperative oxaliplatin +5-FU/capecitabine (n=341) to 5-FU capecitabine alone (n=309). For patients <75 years, the use of post-operative 5-FU/capecitabine demonstrated a reduction in mortality in the post-operative setting but oxaliplatin did not provide additional survival benefit. For patients ages ≥75, there was no mortality reduction for 5-FU/capecitabine (124). In another SEER analysis, investigators found that the use of postoperative oxaliplatin with 5-FU after neoadjuvant chemoradiation and curative resection could prolong survival in those <73 years of age with pathologically positive lymph nodes, but oxaliplatin did not improve survival rates in the postoperative setting for those patients ≥73 years (125). These studies were unable to evaluate the rates or patterns of recurrence based on the nature of the SEER database.
Given the similarities in the use of postoperative chemotherapy for colon cancer, one may consider appraising the data for non-rectal colon cancers and consider extrapolating those data to rectal cancer. Sargent et al. incorporated data from seven phase III randomized trials (n=3,351) and compared the effects of postoperative 5-FU -based therapy (with leucovorin or levamisole) to the effects of surgery alone in resected stages II and III colon cancer. The 5-year overall survival was 71% for those treated with adjuvant therapy versus 64% for surgery alone and the toxicity effects were not increased among those >70 years (126). The addition of oxaliplatin to 5-FU and leucovorin did not show an overall or DFS benefit for patients over 70 years in the NSAPB C-07 study possibly related to higher rates and grades of toxicities compared to younger patients (127). Although 5-FU and oxaliplatin combinations have seemed to demonstrate a limited benefit for elderly patients, a study using the ACCENT database suggested that for patients >70 years, fluoropyrimidine monotherapy with either 5-FU plus leucovorin or capecitabine is an appropriate adjuvant therapy (128). A SEER study linked to the New York State Cancer Registry suggested that the addition of oxaliplatin for stage III colon cancer in patients >75 years offered no more than a small incremental benefit compared to non-oxaliplatin regimens (129). The authors recommended that consideration be made for the use of adjuvant chemotherapy with discussions evaluating individual risks and preferences. Given the variation in underlying medical comorbidities, performance status and pathological risk factors for elderly patients, adjuvant chemotherapy should be considered. Oxaliplatin may increase the toxicity of adjuvant therapy, and as such, may be avoided to provide a more tolerable yet efficacious postoperative chemotherapy regimen using 5-FU therapies with or without leucovorin.
Conclusions
Thus, data for the optimal treatment strategy for rectal cancer in the elderly remains mixed along with significant limitations inherent to retrospective and population-based analyses in the absence of prospective randomized studies.
Based on these recommendations and on the literature review detailed above, a treatment algorithm has been established to help guide the oncologist in treatment decision-making when facing an elderly patient with non-metastatic rectal cancer (Figure 1). For the fit patients with acceptable sphincter tone, standard of care therapy should be employed. For fit patients with unacceptable sphincter tone and cT2+ or N1–2, an APR should be favored. For the class 2 patients with cT3+ or N1–2, both neoadjuvant long-course chemoradiation and short-course radiation are appropriate. Regarding the surgically inoperable or frail patients with more advanced disease, more intensive radiotherapy options could be considered.
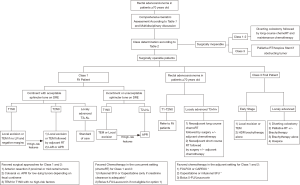
We strongly suggest that the patient undergo a CGA or other multi-dimensional assessment and the case should be discussed at multidisciplinary tumor board with a medical oncologist, GI surgeon, radiation oncologist, physician extenders, nutritionist, nurse and geriatrician. Although it is important to adequately treat the patient, clinicians should attempt to optimize treatment to maximize patient safety and avoid under- or over-treatment. Most studies suggest that elderly patients can tolerate standard courses of therapy with no interruptions; however, clinicians must be vigilant to quickly identify and address toxicities and make appropriate adjustments to therapy, which supports the need for a team approach for care.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [Crossref] [PubMed]
- Hurria A, Dale W, Mooney M, et al. Designing therapeutic clinical trials for older and frail adults with cancer: U13 conference recommendations. J Clin Oncol 2014;32:2587-94. [Crossref] [PubMed]
- Luo R, Giordano SH, Freeman JL, et al. Referral to medical oncology: a crucial step in the treatment of older patients with stage III colon cancer. Oncologist 2006;11:1025-33. [Crossref] [PubMed]
- Abrams TA, Brightly R, Mao J, et al. Patterns of adjuvant chemotherapy use in a population-based cohort of patients with resected stage II or III colon cancer. J Clin Oncol 2011;29:3255-62. [Crossref] [PubMed]
- Schrag D, Gelfand SE, Bach PB, et al. Who gets adjuvant treatment for stage II and III rectal cancer? Insight from surveillance, epidemiology, and end results--Medicare. J Clin Oncol 2001;19:3712-8. [Crossref] [PubMed]
- Papamichael D, Audisio RA, Glimelius B, et al. Treatment of colorectal cancer in older patients: International Society of Geriatric Oncology (SIOG) consensus recommendations 2013. Ann Oncol 2015;26:463-76. [Crossref] [PubMed]
- Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 2005;55:241-52. [Crossref] [PubMed]
- Wildiers H, Heeren P, Puts M, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 2014;32:2595-603. [Crossref] [PubMed]
- Williams GR, Deal AM, Jolly TA, et al. Feasibility of geriatric assessment in community oncology clinics. J Geriatr Oncol 2014;5:245-51. [Crossref] [PubMed]
- Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist 1970;10:20-30. [Crossref] [PubMed]
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179-86. [Crossref] [PubMed]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. [Crossref] [PubMed]
- Bellera CA, Rainfray M, Mathoulin-Pelissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol 2012;23:2166-72. [Crossref] [PubMed]
- Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc 1968;16:622-6. [Crossref] [PubMed]
- Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142-8. [Crossref] [PubMed]
- Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the "get-up and go" test. Arch Phys Med Rehabil 1986;67:387-9. [PubMed]
- Fountoulakis KN, Tsolaki M, Iacovides A, et al. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging (Milano) 1999;11:367-72. [PubMed]
- Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: The Mini Nutritional Assessment as part of the geriatric evaluation. Nutr Rev 1996;54:S59-65. [Crossref] [PubMed]
- Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist 2000;5:224-37. [Crossref] [PubMed]
- Angleman SB, Santoni G, Pilotto A, et al. Multidimensional Prognostic Index in Association with Future Mortality and Number of Hospital Days in a Population-Based Sample of Older Adults: Results of the EU Funded MPI_AGE Project. PLoS One 2015;10:e0133789. [Crossref] [PubMed]
- Volpato S, Bazzano S, Fontana A, et al. Multidimensional Prognostic Index predicts mortality and length of stay during hospitalization in the older patients: a multicenter prospective study. J Gerontol A Biol Sci Med Sci 2015;70:325-31. [Crossref] [PubMed]
- Brunello A, Fontana A, Zafferri V, et al. Development of an oncological-multidimensional prognostic index (Onco-MPI) for mortality prediction in older cancer patients. J Cancer Res Clin Oncol 2016;142:1069-77. [Crossref] [PubMed]
- Giantin V, Falci C, De Luca E, et al. Performance of the Multidimensional Geriatric Assessment and Multidimensional Prognostic Index in predicting negative outcomes in older adults with cancer. Eur J Cancer Care (Engl) 2018.27. [PubMed]
- Chaibi P, Magne N, Breton S, et al. Influence of geriatric consultation with comprehensive geriatric assessment on final therapeutic decision in elderly cancer patients. Crit Rev Oncol Hematol 2011;79:302-7. [Crossref] [PubMed]
- Girre V, Falcou MC, Gisselbrecht M, et al. Does a geriatric oncology consultation modify the cancer treatment plan for elderly patients? J Gerontol A Biol Sci Med Sci 2008;63:724-30. [Crossref] [PubMed]
- Hamaker ME, Jonker JM, de Rooij SE, et al. Frailty screening methods for predicting outcome of a comprehensive geriatric assessment in elderly patients with cancer: a systematic review. Lancet Oncol 2012;13:e437-44. [Crossref] [PubMed]
- Hoppe S, Rainfray M, Fonck M, et al. Functional decline in older patients with cancer receiving first-line chemotherapy. J Clin Oncol 2013;31:3877-82. [Crossref] [PubMed]
- Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377-86. [Crossref] [PubMed]
- Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457-65. [Crossref] [PubMed]
- Lagro J, Studenski SA, Olde Rikkert MG. Predicting chemotherapy toxicity in older adults and the importance of geriatric assessment. J Clin Oncol 2012;30:560-author reply 561-2. [Crossref] [PubMed]
- Hurria A, Levit LA, Dale W, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. J Clin Oncol 2015;33:3826-33. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Swedish Rectal Cancer Trial, Cedermark B, Dahlberg M, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997;336:980-7. [Crossref] [PubMed]
- Folkesson J, Birgisson H, Pahlman L, et al. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol 2005;23:5644-50. [Crossref] [PubMed]
- McCarthy K, Pearson K, Fulton R, et al. Pre-operative chemoradiation for non-metastatic locally advanced rectal cancer. Cochrane Database Syst Rev 2012;12:CD008368. [PubMed]
- Chang GJ, Skibber JM, Feig BW, et al. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg 2007;246:215-21. [Crossref] [PubMed]
- Neugut AI, Fleischauer AT, Sundararajan V, et al. Use of adjuvant chemotherapy and radiation therapy for rectal cancer among the elderly: a population-based study. J Clin Oncol 2002;20:2643-50. [Crossref] [PubMed]
- Jung B, Pahlman L, Johansson R, et al. Rectal cancer treatment and outcome in the elderly: an audit based on the Swedish Rectal Cancer Registry 1995-2004. BMC Cancer 2009;9:68. [Crossref] [PubMed]
- Jiang DM, Raissouni S, Mercer J, et al. Clinical outcomes of elderly patients receiving neoadjuvant chemoradiation for locally advanced rectal cancer. Ann Oncol 2015;26:2102-6. [Crossref] [PubMed]
- Choi Y, Kim JH, Kim JW, et al. Preoperative chemoradiotherapy for elderly patients with locally advanced rectal cancer-a real-world outcome study. Jpn J Clin Oncol 2016;46:1108-17. [PubMed]
- Guimas V, Boustani J, Schipman B, et al. Preoperative Chemoradiotherapy for Rectal Cancer in Patients Aged 75 Years and Older: Acute Toxicity, Compliance with Treatment, and Early Results. Drugs Aging 2016;33:419-25. [Crossref] [PubMed]
- Pasetto LM, Friso ML, Pucciarelli S, et al. Rectal cancer neoadjuvant treatment in elderly patients. Anticancer Res 2006;26:3913-23. [PubMed]
- Bohac GC, Guaqueta D, Cheng DM, et al. Disparity in the use of combined modality therapy for rectal cancer in the older adult. J Geriatr Oncol 2013;4:90-7. [Crossref] [PubMed]
- Moureau-Zabotto L, Resbeut M, Gal J, et al. Management and clinical outcome of rectal cancer in patients >/= 80 years treated in southern France (PACA region) between 2006 and 2008. J Surg Oncol 2013;108:450-6. [Crossref] [PubMed]
- Dharma-Wardene MW, de Gara C, Au HJ, et al. Ageism in rectal carcinoma? Treatment and outcome variations. Int J Gastrointest Cancer 2002;32:129-38. [Crossref] [PubMed]
- Guillerme F, Clavier JB, Nehme-Schuster H, et al. Age impacts the pattern of care for elderly patients with rectal cancer. Int J Colorectal Dis 2014;29:157-63. [Crossref] [PubMed]
- Wan JF, Zhu J, Li GC, et al. Implications for determining the optimal treatment for locally advanced rectal cancer in elderly patients aged 75 years and older. Oncotarget 2015;6:30377-83. [Crossref] [PubMed]
- Thiels CA, Bergquist JR, Meyers AJ, et al. Outcomes with multimodal therapy for elderly patients with rectal cancer. Br J Surg 2016;103:e106-14. [Crossref] [PubMed]
- Dobie SA, Warren JL, Matthews B, et al. Survival benefits and trends in use of adjuvant therapy among elderly stage II and III rectal cancer patients in the general population. Cancer 2008;112:789-99. [Crossref] [PubMed]
- Francois E, Azria D, Gourgou-Bourgade S, et al. Results in the elderly with locally advanced rectal cancer from the ACCOR12/PRODIGE 2 phase III trial: tolerance and efficacy. Radiother Oncol 2014;110:144-9. [Crossref] [PubMed]
- Cai X, Wu H, Peng J, et al. Tolerability and outcomes of radiotherapy or chemoradiotherapy for rectal cancer in elderly patients aged 70 years and older. Radiat Oncol 2013;8:86. [Crossref] [PubMed]
- De Felice F, Musio D, Izzo L, et al. Preoperative chemoradiotherapy in elderly patients with locally advanced rectal cancer. Biomed Res Int 2013;2013:610786. [PubMed]
- Fiorica F, Cartei F, Carau B, et al. Adjuvant radiotherapy on older and oldest elderly rectal cancer patients. Arch Gerontol Geriatr 2009;49:54-9. [Crossref] [PubMed]
- Larsen SG, Wiig JN, Tretli S, et al. Surgery and pre-operative irradiation for locally advanced or recurrent rectal cancer in patients over 75 years of age. Colorectal Dis 2006;8:177-85. [Crossref] [PubMed]
- Lorchel F, Peignaux K, Crehange G, et al. Preoperative radiotherapy in elderly patients with rectal cancer. Gastroenterol Clin Biol 2007;31:436-41. [Crossref] [PubMed]
- Pasetto LM, Basso U, Friso ML, et al. Determining therapeutic approaches in the elderly with rectal cancer. Drugs Aging 2007;24:781-90. [Crossref] [PubMed]
- Di Genesio Pagliuca M, Perotti C, Apicella G, et al. Concurrent chemo-radiotherapy in elderly patients: tolerance and compliance in a series of 137 patients. Clin Transl Oncol 2016;18:571-5. [Crossref] [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215-23. [Crossref] [PubMed]
- Latkauskas T, Pauzas H, Gineikiene I, et al. Initial results of a randomized controlled trial comparing clinical and pathological downstaging of rectal cancer after preoperative short-course radiotherapy or long-term chemoradiotherapy, both with delayed surgery. Colorectal Dis 2012;14:294-8. [Crossref] [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827-33. [Crossref] [PubMed]
- Rutten H, den Dulk M, Lemmens V, et al. Survival of elderly rectal cancer patients not improved: analysis of population based data on the impact of TME surgery. Eur J Cancer 2007;43:2295-300. [Crossref] [PubMed]
- Shahir MA, Lemmens VE, van de Poll-Franse LV, et al. Elderly patients with rectal cancer have a higher risk of treatment-related complications and a poorer prognosis than younger patients: a population-based study. Eur J Cancer 2006;42:3015-21. [Crossref] [PubMed]
- Maas HA, Lemmens VE, Nijhuis PH, et al. Benefits and drawbacks of short-course preoperative radiotherapy in rectal cancer patients aged 75 years and older. Eur J Surg Oncol 2013;39:1087-93. [Crossref] [PubMed]
- Christoforidis D, McNally MP, Jarosek SL, et al. Endocavitary contact radiation therapy for ultrasonographically staged T1 N0 and T2 N0 rectal cancer. Br J Surg 2009;96:430-6. [Crossref] [PubMed]
- Hull TL, Lavery IC, Saxton JP. Endocavitary irradiation. An option in select patients with rectal cancer. Dis Colon Rectum 1994;37:1266-70. [Crossref] [PubMed]
- Kovalic JJ. Endocavitary irradiation for rectal cancer and villous adenomas. Int J Radiat Oncol Biol Phys 1988;14:261-4. [Crossref] [PubMed]
- Papillon J. Intracavitary irradiation of early rectal cancer for cure. A series of 186 cases. Cancer 1975;36:696-701. [Crossref] [PubMed]
- Sun Myint A, Grieve RJ, McDonald AC, et al. Combined modality treatment of early rectal cancer: the UK experience. Clin Oncol (R Coll Radiol) 2007;19:674-81. [Crossref] [PubMed]
- Gerard JP, Romestaing P, Baulieux J, et al. Local curative treatment of rectal cancer by radiotherapy alone. Colorectal Dis 2003;5:442-4. [Crossref] [PubMed]
- Horiot JC, Roth SL, Calais G, et al. The Dijon clinical staging system for early rectal carcinomas amenable to intracavitary treatment techniques. Radiother Oncol 1990;18:329-37. [Crossref] [PubMed]
- Papillon J. Present status of radiation therapy in the conservative management of rectal cancer. Radiother Oncol 1990;17:275-83. [Crossref] [PubMed]
- Schild SE, Martenson JA, Gunderson LL. Endocavitary radiotherapy of rectal cancer. Int J Radiat Oncol Biol Phys 1996;34:677-82. [Crossref] [PubMed]
- Sischy B, Hinson EJ, Wilkinson DR. Definitive radiation therapy for selected cancers of the rectum. Br J Surg 1988;75:901-3. [Crossref] [PubMed]
- Myint AS. Contact radiotherapy for elderly patients with early low rectal cancers. Br J Hosp Med (Lond) 2013;74:391-6. [Crossref] [PubMed]
- Aumock A, Birnbaum EH, Fleshman JW, et al. Treatment of rectal adenocarcinoma with endocavitary and external beam radiotherapy: results for 199 patients with localized tumors. Int J Radiat Oncol Biol Phys 2001;51:363-70. [Crossref] [PubMed]
- Gerard JP, Chapet O, Ramaioli A, et al. Long-term control of T2-T3 rectal adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys 2002;54:142-9. [Crossref] [PubMed]
- Myerson RJ, Hunt SR. Conservative alternatives to extirpative surgery for rectal cancer. Clin Oncol (R Coll Radiol) 2007;19:682-6. [Crossref] [PubMed]
- Corner C, Bryant L, Chapman C, et al. High-dose-rate afterloading intraluminal brachytherapy for advanced inoperable rectal carcinoma. Brachytherapy 2010;9:66-70. [Crossref] [PubMed]
- Clark AJ, Stockton D, Elder A, et al. Assessment of outcomes after colorectal cancer resection in the elderly as a rationale for screening and early detection. Br J Surg 2004;91:1345-51. [Crossref] [PubMed]
- Etzioni DA, Beart RW Jr, Madoff RD, et al. Impact of the aging population on the demand for colorectal procedures. Dis Colon Rectum 2009;52:583-90; discussion 590-1. [Crossref] [PubMed]
- ACS NSQIP hospitals significantly improve outcomes over time. Bull Am Coll Surg 2015;100:64. [PubMed]
- Barrier A, Ferro L, Houry S, et al. Rectal cancer surgery in patients more than 80 years of age. Am J Surg 2003;185:54-7. [Crossref] [PubMed]
- Lemmens VE, Janssen-Heijnen ML, Verheij CD, et al. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg 2005;92:615-23. [Crossref] [PubMed]
- Al-Refaie WB, Parsons HM, Habermann EB, et al. Operative outcomes beyond 30-day mortality: colorectal cancer surgery in oldest old. Ann Surg 2011;253:947-52. [Crossref] [PubMed]
- Janssen-Heijnen ML, Maas HA, Houterman S, et al. Comorbidity in older surgical cancer patients: influence on patient care and outcome. Eur J Cancer 2007;43:2179-93. [Crossref] [PubMed]
- Kiran RP, Pokala N, Dudrick SJ. Long-term outcome after operative intervention for rectal cancer in patients aged over 80 years: analysis of 9,501 patients. Dis Colon Rectum 2007;50:604-10. [Crossref] [PubMed]
- Singh J, Stift A, Brus S, et al. Rectal cancer surgery in older people does not increase postoperative complications--a retrospective analysis. World J Surg Oncol 2014;12:355. [Crossref] [PubMed]
- Tan KK, Koh FH, Tan YY, et al. Long-term outcome following surgery for colorectal cancers in octogenarians: a single institution's experience of 204 patients. J Gastrointest Surg 2012;16:1029-36. [Crossref] [PubMed]
- Bhangu A, Rasheed S, Brown G, et al. Does rectal cancer height influence the oncological outcome? Colorectal Dis 2014;16:801-8. [Crossref] [PubMed]
- Buess G, Theiss R, Gunther M, et al. Endoscopic surgery in the rectum. Endoscopy 1985;17:31-5. [Crossref] [PubMed]
- Cataldo PA. Transanal endoscopic microsurgery. Surg Clin North Am 2006;86:915-25. [Crossref] [PubMed]
- Christoforidis D, Cho HM, Dixon MR, et al. Transanal endoscopic microsurgery versus conventional transanal excision for patients with early rectal cancer. Ann Surg 2009;249:776-82. [Crossref] [PubMed]
- Saclarides TJ. TEM/local excision: indications, techniques, outcomes, and the future. J Surg Oncol 2007;96:644-50. [Crossref] [PubMed]
- Tsai BM, Finne CO, Nordenstam JF, et al. Transanal endoscopic microsurgery resection of rectal tumors: outcomes and recommendations. Dis Colon Rectum 2010;53:16-23. [Crossref] [PubMed]
- Floyd ND, Saclarides TJ. Transanal endoscopic microsurgical resection of pT1 rectal tumors. Dis Colon Rectum 2006;49:164-8. [Crossref] [PubMed]
- De Graaf EJ, Doornebosch PG, Tollenaar RA, et al. Transanal endoscopic microsurgery versus total mesorectal excision of T1 rectal adenocarcinomas with curative intention. Eur J Surg Oncol 2009;35:1280-5. [Crossref] [PubMed]
- Jeong WK, Park JW, Choi HS, et al. Transanal endoscopic microsurgery for rectal tumors: experience at Korea's National Cancer Center. Surg Endosc 2009;23:2575-9. [Crossref] [PubMed]
- Lee W, Lee D, Choi S, et al. Transanal endoscopic microsurgery and radical surgery for T1 and T2 rectal cancer. Surg Endosc 2003;17:1283-7. [Crossref] [PubMed]
- Lu JY, Lin GL, Qiu HZ, et al. Comparison of Transanal Endoscopic Microsurgery and Total Mesorectal Excision in the Treatment of T1 Rectal Cancer: A Meta-Analysis. PLoS One 2015;10:e0141427. [Crossref] [PubMed]
- Sengupta S, Tjandra JJ. Local excision of rectal cancer: what is the evidence? Dis Colon Rectum 2001;44:1345-61. [Crossref] [PubMed]
- Garcia-Aguilar J, Renfro LA, Chow OS, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 2015;16:1537-46. [Crossref] [PubMed]
- Verseveld M, de Graaf EJ, Verhoef C, et al. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg 2015;102:853-60. [Crossref] [PubMed]
- Heald RJ, Moran BJ, Ryall RD, et al. Rectal cancer: the Basingstoke experience of total mesorectal excision, 1978-1997. Arch Surg 1998;133:894-9. [Crossref] [PubMed]
- Kapiteijn E, Putter H, van de Velde CJ, et al. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 2002;89:1142-9. [Crossref] [PubMed]
- Rutten HJ, den Dulk M, Lemmens VE, et al. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol 2008;9:494-501. [Crossref] [PubMed]
- Clinical Outcomes of Surgical Therapy Study G. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Lujan J, Valero G, Hernandez Q, et al. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg 2009;96:982-9. [Crossref] [PubMed]
- van der Pas MH, Haglind E, Cuesta MA, et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Akiyoshi T, Kuroyanagi H, Oya M, et al. Short-term outcomes of laparoscopic rectal surgery for primary rectal cancer in elderly patients: is it safe and beneficial? J Gastrointest Surg 2009;13:1614-8. [Crossref] [PubMed]
- Manceau G, Hain E, Maggiori L, et al. Is the benefit of laparoscopy maintained in elderly patients undergoing rectal cancer resection? An analysis of 446 consecutive patients. Surg Endosc 2017;31:632-42. [Crossref] [PubMed]
- Otsuka K, Kimura T, Hakozaki M, et al. Comparative benefits of laparoscopic surgery for colorectal cancer in octogenarians: a case-matched comparison of short- and long-term outcomes with middle-aged patients. Surg Today 2017;47:587-94. [Crossref] [PubMed]
- Roscio F, Boni L, Clerici F, et al. Is laparoscopic surgery really effective for the treatment of colon and rectal cancer in very elderly over 80 years old? A prospective multicentric case-control assessment. Surg Endosc 2016;30:4372-82. [Crossref] [PubMed]
- Fleshman J, Branda M, Sargent DJ, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA 2015;314:1346-55. [Crossref] [PubMed]
- Stevenson AR, Solomon MJ, Lumley JW, et al. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- Bedirli A, Salman B, Yuksel O. Robotic Versus Laparoscopic Resection for Mid and Low Rectal Cancers. JSLS 2016;20(1).
- Moghadamyeghaneh Z, Phelan M, Smith BR, et al. Outcomes of Open, Laparoscopic, and Robotic Abdominoperineal Resections in Patients With Rectal Cancer. Dis Colon Rectum 2015;58:1123-9. [Crossref] [PubMed]
- Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin 2010;60:120-32. [Crossref] [PubMed]
- Engel J, Kerr J, Schlesinger-Raab A, et al. Quality of life in rectal cancer patients: a four-year prospective study. Ann Surg 2003;238:203-13. [Crossref] [PubMed]
- Dehni N, Tiret E, Singland JD, et al. Long-term functional outcome after low anterior resection: comparison of low colorectal anastomosis and colonic J-pouch-anal anastomosis. Dis Colon Rectum 1998;41:817-22; discussion 822-3. [Crossref] [PubMed]
- Hida J, Yoshifuji T, Okuno K, et al. Long-term functional outcome of colonic J-pouch reconstruction after low anterior resection for rectal cancer. Surg Today 2006;36:441-9. [Crossref] [PubMed]
- Phillips PS, Farquharson SM, Sexton R, et al. Rectal cancer in the elderly: patients' perception of bowel control after restorative surgery. Dis Colon Rectum 2004;47:287-90. [Crossref] [PubMed]
- Margalit DN, Mamon HJ, Ancukiewicz M, et al. Tolerability of combined modality therapy for rectal cancer in elderly patients aged 75 years and older. Int J Radiat Oncol Biol Phys 2011;81:e735-41. [Crossref] [PubMed]
- Lund JL, Sturmer T, Sanoff HK. Comparative effectiveness of postoperative chemotherapy among older patients with non-metastatic rectal cancer treated with preoperative chemoradiotherapy. J Geriatr Oncol 2016;7:176-86. [Crossref] [PubMed]
- Huang XZ, Gao P, Song YX, et al. Impact of age on efficacy of postoperative oxaliplatin-based chemotherapy in patients with rectal cancer after neoadjuvant chemoradiotherapy. Oncotarget 2016;7:19643-53. [PubMed]
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091-7. [Crossref] [PubMed]
- Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768-74. [Crossref] [PubMed]
- McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600-6. [Crossref] [PubMed]
- Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624-34. [Crossref] [PubMed]


