Comparison of perioperative chemotherapy with adjuvant chemoradiotherapy for resectable gastric cancer: findings from a population-based study
Introduction
Worldwide, gastric cancer is the 5th most frequent cancer and the second leading cause of cancer deaths (1). In the United States (US), gastric cancer mortality has been declining since 1930 (2), currently representing less than two percent of all new cancer cases (3). Most US gastric cancer patients present with loco-regionally advanced (28%) or metastatic disease (35%), contributing to the observed 5-year overall survival (OS) of 31% and 5%, respectively (4). Although surgery is the mainstay of treatment for patients with gastric cancer, multimodal treatment is required for most patients to achieve long-term survival (5,6).
In 2001, findings from the Southwestern Oncology Group-Intergroup Trial (INT0116) in the US demonstrated that adjuvant chemoradiotherapy (CRT) with fluorouracil and leucovorin plus external-beam radiation was associated with improved OS compared to surgery-only (7). In this trial, subjects in the CRT treatment arm had a median survival of 36 vs. 27 months for surgery-only; P=0.005, with an overall mortality hazards ratio (HR) of 0.74 (95% CI: 0.60–0.92) (7). Subsequently, the Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial in the United Kingdom (UK), showed that administration of perioperative chemotherapy (PC) with epirubicin, cisplatin, and fluorouracil resulted in improved OS compared to surgery-only (HR =0.75; 95% CI: 0.60–93) (8). The INT0116 and MAGIC trials established CRT and PC as two evidence-based standards of care for resectable gastric cancer (9,10), with adoption of CRT as the recommended treatment in the US (9) and with PC preferred in the UK and other European countries (10).
More recently, CRT and PC have both been included in the US National Comprehensive Cancer Network (NCCN) treatment guidelines for resectable gastric cancer (11). However, PC and CRT have never been directly compared in a clinical trial. Additionally, ongoing gastric cancer clinical trials that include the trial of Preoperative Therapy For Gastric And Esophagogastric Junction Adenocarcinoma (TOPGEAR) (11), Randomized phase III trial of Adjuvant Chemotherapy Or Chemoradiotherapy In Resectable Gastric Cancer (CRITICS) trial (10), and Adjuvant Chemoradiation Therapy In Stomach Cancer (ARTIST-2) trial (12), are not designed to compare survival differences between PC and adjuvant CRT. Given that PC and adjuvant CRT represent the most commonly used treatment protocols for resectable gastric cancer in the West, we sought to use California Cancer Registry (CCR) data to contrast survival outcomes among patients receiving these two treatment protocols.
Methods
Study population
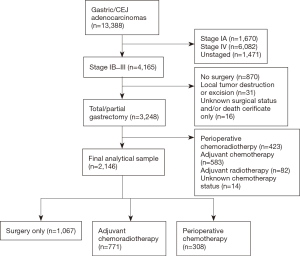
The CCR, consisting of the three most populated Surveillance Epidemiology and End Results (SEER) program registries of the US, is the statewide cancer surveillance system that has continuously collected data on cancer occurrence, treatment, and mortality in California since 1988 (13). Using CCR data, patients diagnosed with stage Ib–III (14) gastric and gastroesophageal junction (GEJ) adenocarcinoma (M-8120-M-8240 and M-8255-M-8576) (15) were identified (Figure 1). This study was conducted using existing data, without patient contact. Study methods were approved by the Loma Linda University Institutional Review Board. Consistent with the MAGIC trial, patients were classified as having received PC if chemotherapy was initiated before surgery, regardless of whether postoperative chemotherapy was administered. Patients that received a combination of chemotherapy and radiotherapy following surgery were classified as receiving CRT. Additionally, using the CCR Eureka database system, which is exclusive to the CCR, patient-level imaging report text fields were visually reviewed to derive clinical node status information. Three clinical node status categories were formed as positive (CN-positive), negative (CN-negative) and unknown (CN-unknown). Patients were classified as having CN-positive disease if any of the following criteria were stated in the patient’s imaging report: computerized tomography scans showing node(s) greater than 1 cm; positron emission tomography imaging having hypermetabolic nodes; or endoscopic ultrasound report of node(s) greater than 1 cm, findings of hypoechoic nodes, findings specifically described as ultrasound node positive (uN+) or otherwise stated as “suspicious”. Patients were classified as having CN-negative disease if their imaging report stated “negative” findings. Otherwise, clinical node status was classified as “CN-unknown”.
OS was the primary outcome, while gastric cancer specific survival (GCSS) was secondary. Patient survival time was calculated as the period extending from the date of surgery to death or to last date of study follow-up (December 31, 2014), whichever occurred first. Demographic covariates included age (<60, 60–69, 70+), sex (female or male), race/ethnicity (Asian/other, Hispanic, non-Hispanic black, non-Hispanic white) and year of diagnosis [2007–2013]. Postoperative tumor characteristics that were adjusted in the study included T-stage (1–2 and 3–4) (14); histology type as intestinal (M-8144), diffuse (M-8145) or mixed; signet ring (M-8490) (15); extent of lymphadenectomy (<15, 15–25, 26+ lymph nodes) (16,17); and proximal tumor location (yes/no).
Statistical analyses
Patient and tumor characteristics were summarized using counts and percentages with comparisons between treatment cohorts conducted using χ2 tests for independence. Kaplan-Meier survival curves and log-rank tests were used to compare median survival times between treatment groups, while median follow-up time was estimated using the inverse Kaplan-Meier curve (18). Propensity score adjustment was used to balance demographic characteristic differences between treatment cohorts using a two-stage process (1). Initially, logistic regression was used to predict the probability of receiving treatment using age, sex, race/ethnicity and year of diagnosis as covariates in the model. Subsequently, using inverse propensity score weighting (IPWT), IPWT-Cox proportional hazards regression models that adjusted for postoperative tumor characteristics were used to calculate weighted mortality HRs. Inverse propensity score weighted analyses were conducted for the comparison of each treatment pair and all P values after adjustments were found to be <0.05. Proportionality assumptions were evaluated using log-log plots and Schoenfeld residuals. Additionally, the Cochran-Armitage trend test was used to assess linear changes in treatment patterns during the study period. All tests were two-sided, conducted using a significance level of five percent (α=0.05), and were performed using SAS software, version.9.4 of the SAS system for Windows. Copyright© 2002–2012 SAS Institute Inc., Cary, NC, USA.
Subgroup analyses
All analyses were conducted within homogeneous clinical lymph node strata. Additionally, to assess the effect of PC and CRT on signet ring cell histology, subgroup analyses were conducted on patients with signet ring histology.
Results
We identified, 13,388 patients diagnosed with gastric/GEJ adenocarcinoma during the study period. Patients with stage IA or IV disease, having inadequate staging or treatment information, lacking surgical resection, or who received other combinations of adjuvant treatment were excluded. The final study population of 2,146 patients included 1,067 treated with surgery-only, 771 with CRT, and 308 with PC (Figure 1).
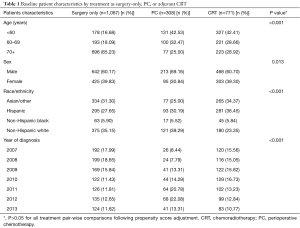
Patient demographic characteristics are described in Table 1. The majority of the patients in the surgery-only group were age 70 years or older (65.23%), while more than 70% of patients in both PC and adjuvant CRT groups were less than age 70 years at diagnosis; P<0.001. More than 60% of patients in each treatment group were males; P=0.013. Non-Hispanic white patients were more likely to be treated with surgery-only (35.15%) or PC (39.29%), while CRT was more often utilized among Hispanic (36.45%) and Asian/other patients (34.37%); P<0.001. Initial differences observed in demographic characteristic distributions where substantially eliminated by IPWT adjustment (Table 1). There were no significant differences in American College of Surgeons Commission on Cancer accreditation (yes/no) for surgery-only, PC and CRT cohorts; P=0.143.
Full table
Tumor and treatment characteristics
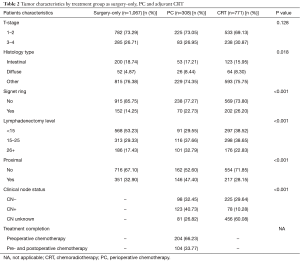
The majority of patients were diagnosed with T1–T2 tumors, with no significant difference seen between treatment groups; P=0.128. Prevalence of diffuse histology was comparable for patients receiving PC (8.44%) and CRT (8.30%), with slightly lower prevalence seen for surgery-only patients (4.87%); P=0.018. Similarly, prevalence of signet ring histology was slightly higher in PC (22.73%) and CRT (26.20%) patients, compared to surgery-only (14.25%); P<0.001. Fifteen or more lymph nodes were examined in 70.45% of PC, 61.48% of CRT and 46.77% of surgery-only patients; P<0.001. Proximal tumors were more frequent in PC (47.40%) than adjuvant CRT (28.15%) or surgery-only (32.90%) patients; P<0.001. Clinical node status was known for 73.18% of PC patients and 39.92% of CRT patients; P<0.001. Only 33.8% of PC patients received any postoperative chemotherapy (Table 2).
Full table
PC and CRT vs. surgery-only
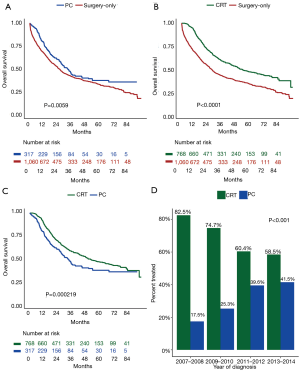
The overall median follow-up time for all study subjects, regardless of extent of lymphadenectomy, was 56 months. Additionally, median survival (Figure 2) was significantly longer for: PC (33 months) vs. surgery-only (25 months) (Figure 2A), CRT (52 months) vs. surgery-only (25 months) (Figure 2B) and CRT vs. PC (Figure 2C). Among all study subjects, survival was significantly longer for both PC (HR =0.75; 95% CI: 0.65–0.87) and CRT (HR =0.62; 95% CI: 0.54–0.70), compared to surgery-only, with similar findings among patients having 15 or more lymph nodes dissected (Figure 3).
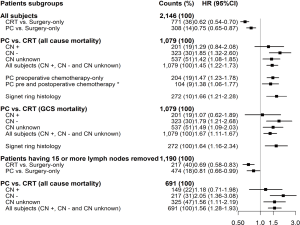
All-cause mortality for PC vs. CRT
Overall, patients treated with PC had higher mortality compared to CRT (HR =1.45; 95% CI: 1.22–1.73). Further comparison by clinical node status revealed that CN-negative patients treated with PC had significantly higher mortality (HR =1.85; 95% CI: 1.32–2.60), compared to patients treated with adjuvant CRT. For CN-positive patients, there was no significant difference in mortality between the two treatment groups (HR =1.29; 95% CI: 0.84–2.08). Since only 34% of PC patients received any postoperative chemotherapy, separate comparisons were performed for pre- and post-operative chemotherapy categories. Mortality risk for pre- and post-operative chemotherapy, compared to CRT, were HR =1.47; 95% CI: 1.23–1.78 and HR =1.38; 95% CI: 1.06–1.77, respectively, with no significant difference observed between the two PC groups; P=0.597. Furthermore, among patients with signet ring histology, OS was significantly poorer for PC compared to CRT (HR =1.66; 95% CI: 1.21–2.28). Gastric cancer-specific mortality findings were similar to those obtained for all-cause mortality (Figure 3).
PC vs. CRT among patients having adequate lymphadenectomy
Findings for PC vs. CRT among patients that had 15 or more lymph nodes dissected (HR =1.56; 95% CI: 1.28–1.93) were similar to those for all patients, regardless of lymph node count (Figure 3). Additionally, the contrast of PC vs. CRT among CN-positive patients did not identify significant difference (HR =1.18; 95% CI: 0.71–1.98), although the same contrast made among CN-negative patients revealed higher mortality hazards for the PC cohort (HR =2.05; 95% CI: 1.36–3.08).
PC and CRT treatment patterns
PC accounted for 17.5%, 25.3%, 39.6%, and 41.5% of all patients that received chemotherapy in our study population for years 2007–2008, 2009–2010, 2011–2012, and 2013–2014, respectively. The Cochrane-Armitage test for linear trend showed a P value less than 0.001 (Figure 2D).
Discussion
Current treatment recommendations for resectable gastric cancer in Western countries for adjuvant CRT or PC are based on the INT0116 (7) and MAGIC (8) trials. To date, CRT and PC have not been directly compared in a clinical trial. Given the unmet need to compare PC and CRT, we conducted a large, population-based study comparing these treatment approaches in patients with resected gastric cancer.
In an effort to minimize limitations encountered comparing clinical trial and observational study findings, we restricted study eligibility criteria to correspond with enrolment conditions used in the INT0116 (7) and MAGIC (8) trials. Additionally, propensity scores were used to balance differences in demographic characteristics between the PC and CRT cohorts. To the best of our knowledge, this is the first US-based study to compare PC with CRT.
Similar to the results of the MAGIC and INT0116 trials, we observed survival improvement with addition of either PC or CRT in surgically treated patients. The observed survival benefit of PC in the current study (HR =0.75; 95% CI: 0.65–0.87) was remarkably similar to that found in the MAGIC trial (HR =0.74; 95% CI: 0.59–0.93), with similar findings seen for patients that had 15 or more lymph nodes removed (HR =0.81; 95% CI: 0.66–0.99), validating our findings. Additionally, the survival benefit observed for CRT versus surgery among all subjects (HR =0.62; 95% CI: 0.54–0.70) and for patients that had adequate lymphadenectomy (HR =0.69; 95% CI: 0.58–0.83), was similar, but slightly stronger than the INT0116 trial finding (HR =0.74; 95% CI: 0.54–0.81) (7). There are several explanations for the improved survival observed with CRT in our study. The majority of CRT patients (61.48%) in our study had adequate lymph node evaluation (≥15 nodes) (9). In contrast, the majority of patients (54%) in the INT0166 trial underwent a D0 lymphadenectomy (7). Additionally, improvement in radiation therapy delivery modalities (19) and changes in chemotherapy regimens (20) during recent years might have contributed to improved outcomes observed for CRT. It has also been argued that the benefits of CRT observed in INT0166 trial resulted from adjuvant therapy merely compensating for inadequate surgery (21). Our results refute this assertion, since CRT is associated with significant survival benefit compared to PC and surgery, even among patients with adequate lymphadenectomy. Additionally, our results are in agreement with prior findings reported by Kim et al., which showed longer survival for CRT, compared to surgery-only, among South Korean patients that had undergone D2 lymphadenectomy (22).
The Adjuvant Chemoradiation Therapy in Stomach Cancer (ARTIST) trial, from South Korea, attempted to address the utility of radiotherapy following “adequate surgery”. This study compared, postoperative chemotherapy with or without the addition of radiotherapy in patients undergoing D2 gastrectomy. In this trial, 3-year disease-free survival was not significantly different between the two treatment arms (P=0.08). However, addition of radiotherapy improved survival in an unplanned subgroup analysis of node-positive patients. Since our objective was to compare PC with CRT, patients who were treated with only postoperative chemotherapy without radiation were not included in our study.
Overall comparison of patients treated with PC versus CRT revealed that PC predicted poorer survival compared to CRT among all study subjects (HR =1.45; 95% CI: 1.22–1.73) and among patients having adequate lymphadenectomy (15+ lymph nodes removed) (HR =1.56; 95% CI: 1.28–1.93). One of the unique aspects of our study is the comparison of treatments based on clinical node status. Since, lymph node status is an important predictor for gastric cancer survival; however, potential down staging following PC renders final pathological lymph node status inadequate for comparison of survival benefits for PC and CRT. For this reason, we stratified patients based on clinical nodal status (positive, negative, and unknown). Although there was no difference in OS for PC vs. CRT in CN-positive patients, those with CN-negative disease had significantly higher morality if they had been treated with PC rather than CRT (HR =1.85; 95% CI: 1.32–2.60). This effect was stronger when analyses were limited to patients that had 15 or more lymph nodes removed (HR =2.05; 95% CI: 1.36–3.08).
This is one of the key observations of our study. The observed survival differences between these two treatment approaches warrant strong consideration of CN status in treatment decisions, favoring CRT in CN-negative patients (22).
Only 34% of patients in the PC treatment group received any postoperative chemotherapy. The proportion of patients receiving postoperative chemotherapy in this report is lower than that in the MAGIC trial in which 58% of subjects assigned to the PC arm received postoperative chemotherapy, with 42% completing all six chemotherapy cycles. Nevertheless, receipt of postoperative chemotherapy did not impact the observed outcome differences between PC and CRT in our study. While there may be benefits of PC that include tumor down staging (23,24), earlier treatment of micrometastases (25,26), and appraisal of tumor response to chemotherapy (8), these benefits are arguably more beneficial to patients with CN-positive disease. Delaying definitive surgery, for PC delivery, among the CN-negative patient may explain some of the survival differences observed among CN-negative patients. This explanation is consistent with poorer survival among subjects having adequate surgery.
Another, major observation was that PC was associated with significantly higher mortality in patients with signet ring histology compared to CRT (HR =1.66; 95% CI: 1.21–2.28). This finding is particularly relevant because of the increasing incidence of signet ring and diffuse gastric cancer in Western countries (27,28) and emerging evidence of chemoresistance in this subtype (29,30). Messager et al. conducted a multicenter comparative study of patients with esophagogastric signet ring adenocarcinoma (n=924) treated with primary surgery (n=753) or pre-operative chemotherapy (n=171) (31). Pre-operative chemotherapy was an independent predictor of poorer survival (HR =1.4; 95% CI: 1.1–1.9), P=0.042); OS at 2 years was 12.3% in the preoperative chemotherapy group compared to 27.1% in the primary surgery group. The authors concluded that delaying definitive surgery for ineffective treatment likely contributed to the observed survival difference (31). Based on results of this study, a prospective randomized controlled trial to evaluate treatments strategy in signet ring gastric cancer is ongoing. PRODIGE 19-FFCD1103-ADC1002 is a phase II/III multicenter randomized controlled trial designed to compare standard PC (three cycles of epirubicin, cisplatin and 5-fluorouracil pre- and post-surgery) with primary surgery followed by adjuvant chemotherapy in patients with stage IB–III gastric signet ring cancer (29).
Our findings may have been influenced by biases inherent to observational studies. To address selection bias for patients that may have received one treatment over another, differences in baseline demographic characteristics between treatment groups were balanced using propensity score (inverse probability) weighting (32). Secondly, while information on functional status, American Society of Anesthesiologists physical classification score, or nutrition status was not available, selecting patients fit for a major operation minimized heterogeneity between PC and CRT cohorts. Furthermore, since survival was calculated from the date of surgery, patients in the PC treatment group were defacto, required to survive preoperative chemotherapy and to be clinically fit for surgery. This survival precondition for PC, that does not exist for CRT, creates immortal-time bias (28) in the PC group. Nevertheless, this bias predicts improved survival for PC, relative to CRT, contrary to our findings.
Conclusions
Our study has shown important outcome differences between CRT and PC based on clinical nodal status. PC is associated with poorer survival compared to CRT, in patients with clinical node negative and signet ring cancers, regardless of the extent of lymphadenectomy. In the absence of head to head comparison of CRT versus PC, the results of our study challenge the current treatment recommendations for the use of PC in clinical node negative patients. The relevance of this observation is underscored by rapid adoption of PC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Study methods were approved by the Loma Linda University Institutional Review Board (No. 59061). The IRB approved a waiver of updated consent form, since the analysis was limited to existing data.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. Global Burden of Disease Cancer C. The global burden of cancer 2013. JAMA Oncology 2015;1:505-27. [Crossref] [PubMed]
- Wingo PA, Cardinez CJ, Landis SH, et al. Long-term trends in cancer mortality in the United States, 1930-1998. Cancer 2003;97:3133-275. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Howlader N NA, Krapcho M, Garshell J, et al. editors. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute, 1975-2013.
- Ito H, Clancy TE, Osteen RT, et al. Adenocarcinoma of the gastric cardia: what is the optimal surgical approach? J Am Coll Surg 2004;199:880-6. [Crossref] [PubMed]
- Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317-28. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2016;14:1286-312. [Crossref] [PubMed]
- Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v38-49. [Crossref] [PubMed]
- Leong T, Smithers BM, Michael M, et al. TOPGEAR: a randomised phase III trial of perioperative ECF chemotherapy versus preoperative chemoradiation plus perioperative ECF chemotherapy for resectable gastric cancer (an international, intergroup trial of the AGITG/TROG/EORTC/NCIC CTG). BMC Cancer 2015;15:532. [Crossref] [PubMed]
- Park SH, Lee SJ, Kim ST, et al. Multicenter phase III trial of adjuvant chemoradiotherapy in stomach tumors 2 (ARTIST 2). J Clin Oncol 2015;33:TPS228. [Crossref]
- California Department of Public Health. California Cancer Registry, California Department of Public Health, 2014. Available online: http://www.ccrcal.org/index.shtml
- Greene FL, American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging handbook: from the AJCC cancer staging manual. 6th ed. New York: Springer, 2002.
- Fritz A, Percy C, Jack A, et al. editors. International classification of diseases for oncology: ICD-O. 3rd ed. Malta: World Health Organization, 2013.
- Kim YI. Is retrieval of at least 15 lymph nodes sufficient recommendation in early gastric cancer? Ann Surg Treat Res 2014;87:180-4. [Crossref] [PubMed]
- Baiocchi GL, Tiberio GA, Minicozzi AM, et al. A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg 2010;252:70-3. [Crossref] [PubMed]
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials 1996;17:343-6. [Crossref] [PubMed]
- McCloskey SA, Yang GY. Benefits and Challenges of Radiation Therapy in Gastric Cancer: Techniques for Improving Outcomes. Gastrointest Cancer Res 2009;3:15-9. [PubMed]
- Kilic L, Ordu C, Yildiz I, et al. Current adjuvant treatment modalities for gastric cancer: From history to the future. World J Gastrointest Oncol 2016;8:439-49. [Crossref] [PubMed]
- Scartozzi M, Galizia E, Graziano F, et al. Over-D1 dissection may question the value of radiotherapy as a part of an adjuvant programme in high-risk radically resected gastric cancer patients. Br J Cancer 2005;92:1051-4. [Crossref] [PubMed]
- Kim S, Lim DH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys 2005;63:1279-85. [Crossref] [PubMed]
- D'Ugo D, Persiani R, Rausei S, et al. Response to neoadjuvant chemotherapy and effects of tumor regression in gastric cancer. Eur J Surg Oncol 2006;32:1105-9. [Crossref] [PubMed]
- Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521-30. [Crossref] [PubMed]
- Eguchi T, Kodera Y, Nakanishi H, et al. The effect of chemotherapy against micrometastases and isolated tumor cells in lymph nodes: an in vivo study. In Vivo 2008;22:707-12. [PubMed]
- Wang D, Smit JK, Zwaan E, et al. Neoadjuvant therapy reduces the incidence of nodal micrometastases in esophageal adenocarcinoma. Am J Surg 2013;206:732-8. [Crossref] [PubMed]
- Wu H, Rusiecki JA, Zhu K, et al. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev 2009;18:1945-52. [Crossref] [PubMed]
- Henson DE, Dittus C, Younes M, et al. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med 2004;128:765-70. [PubMed]
- Piessen G, Messager M, Le Malicot K, et al. Phase II/III multicentre randomised controlled trial evaluating a strategy of primary surgery and adjuvant chemotherapy versus peri-operative chemotherapy for resectable gastric signet ring cell adenocarcinomas – PRODIGE 19 – FFCD1103 – ADCI002. BMC Cancer 2013;13:281. [Crossref] [PubMed]
- Voron T, Messager M, Duhamel A, et al. Is signet-ring cell carcinoma a specific entity among gastric cancers? Gastric Cancer 2016;19:1027-40. [Crossref] [PubMed]
- Messager M, Lefevre JH, Pichot-Delahaye V, et al. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg 2011;254:684-93; discussion 693. [Crossref] [PubMed]
- Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661-79. [Crossref] [PubMed]