Contrast enhanced ultrasound guided biopsies of liver lesions not visualized on standard B-mode ultrasound—preliminary experience
Introduction
Distinguishing benign from malignant focal liver lesions (FLL) is crucial in determining prognosis and patient management (1). Although FLL can be characterized to a certain extent based on ultrasound and cross-sectional imaging (2), histological pathology from tissue biopsy remains the diagnostic gold standard to determine patient management (3-6). Furthermore, subsequent management with targeted cell therapy may require tissue acquisition for genetics analysis. Imaging modalities used for guidance of liver biopsies are primarily B-mode ultrasound (US) and computed tomography (CT). B-mode US is the preferred modality for image guided liver lesion biopsy. Compared to US, CT is less frequently used for visualization during biopsy due to concerns about radiation exposure, lack of real time imaging which extends procedure time, and increased costs (7). However, guidance by B-mode US can be challenging due to lack of visualization of FLL that are slightly hyperechoic, hypoechoic or isoechoic to surrounding liver parenchyma. In such cases, B-mode US guided hepatic biopsy has been shown to produce low diagnostic yield (1,8-10) and per current standard these patients need to undergo a CT guided procedure.
Contrast enhanced ultrasound (CEUS) is an evolving technique and involves intravenous administration of microbubble contrast agents which has the potential to improve real-time US guided FLL characterization and biopsy. Studies have demonstrated that CEUS is capable of increasing sensitivity and specificity in detection of malignant hepatic masses compared to B-mode US, citing its advantages in capturing liver perfusion phase patterns to better delineate lesion borders from the surrounding parenchyma (11-17). This led to approval of one US contrast agent for characterization of FLL in adult as well as pediatric patients by the U.S. Food and Drug Administration in April 2016 (18). A very limited number of studies however have investigated the value of CEUS guided procedures. The major potential benefits of CEUS in comparison to B-mode US are higher detection rate of poorly visible lesions, decreased number of samples required and avoidance of sampling necrotic areas of larger liver lesions (1,19-21).
In this retrospective analysis we report our institutional experience with CEUS guided liver biopsy of FLL not well visualized on B-mode US. The patients were selected based on a real-time clinical scenario during the procedure when lesions were poorly visualized on standard B-mode ultrasound. These patients would have otherwise been rescheduled for a CT guided procedure. The purpose of the study was to assess the technical success of CEUS guided biopsies of liver lesions poorly visualized on B-mode ultrasound.
Methods
Subjects
This retrospective study was approved by the local institutional review board of our hospital and the study was HIPAA compliant. The charts and images of 26 patients that underwent CEUS guided liver biopsy were systematically reviewed. All the patients were selected for the procedure based on prior FLL characterization on cross-sectional imaging demonstrating suspicious features (contrast CT or MRI). They had suboptimal lesion visualization on initial B-mode US and met clinical criteria to undergo CEUS guided liver lesion biopsy based on a real-time clinical scenario. These were all patients who were brought back to the ultrasonography procedure room and if CEUS guided biopsy would have not been available, these patients would have had to be rescheduled for CT guided biopsy on another day. Exclusion criteria included less than 2 months of follow-up due to inability to determine technical success rate and cases in which the entire procedure was performed under fusion of US with CT or MRI without substantial involvement of CEUS guidance.
CEUS guided biopsy
CEUS guided biopsy was performed under moderate conscious sedation using Fentanyl and Versed or under general anesthesia. In all patients, either Definity (Lantheus Medical Imaging; composed of perflutren lipid microspheres) or Lumason (Bracco Imaging; composed of sulfur hexafluoride lipid microspheres) were injected intravenously as a single bolus within approximately 20 seconds, followed by a 10 mL saline flush through a peripheral upper extremity vein. The dosage of contrast material ranged between 0.8 and 1.0 mL for Definity or between 2.4 and 4.8 mL for Lumason. After lesion localization, biopsy was performed using a 17 G co-axial system with an 18 G Temno Evolution biopsy device (Allegiance, McGaw Park, IL, USA).
Study parameters
Each case was analyzed individually in regards to the following parameters: demographic information, contrast material used, lesion size and location, FLL appearance on standard B-mode US and on CEUS, procedural technical details, the pathology outcome and technical success. The appearance on CEUS is related to the delayed phase since this phase is crucial when performing CEUS guided procedures. Success was defined as conclusive histopathological diagnosis concordant with imaging appearance and clinical follow-up—without need for re-biopsy, or re-biopsy with identical pathological results.
Results
Twenty-six patients were included in our study, 16 males and 10 females. The average age was 62.1 (±17.3) years. Seven patients (26.9%) had cirrhosis due to different etiologies. All of the patients had clinical follow-up of at least 2 months and the mean follow-up period was 7.1 (±4.6) months. The average procedural time recorded was 30.7 (±12.3) minutes. All patients tolerated contrast agent administration well without side effects. Definity was used as contrast material in 19 patients (73%). The remaining seven patients (27%) received Lumason. The mean number of samples taken from each lesion was 3.2 (±1.7).
Eleven lesions (42.3%) were invisible on B-mode US and the remaining lesions were poorly visualized. Twenty-one of the lesions (80.8%) were predominantly hypoenhancing on the delayed phase of CEUS. The average lesion largest dimension was 2.2 (±1.7) cm. In 18 patients (69.2%) the lesion diameter was smaller than 2 cm. Demographic data of the patients and lesion characteristics are listed in Table 1.
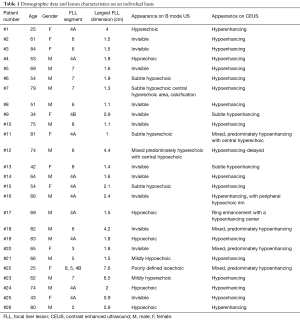
Full table
Pathology results revealed a malignant neoplasm (HCC or metastasis) in 15 cases (57.7%). The pathology outcome of the patients is listed in Table 2. Procedural success was achieved in 23 cases (88.5%), demonstrating concordance between the pathology results and the clinical and imaging follow-up. In the three unsuccessful cases, the pathology result showed no evidence of malignancy but the follow-up imaging or repeated biopsy procedure revealed different findings. Thus in these three cases a re-biopsy was needed and therefore CEUS guided biopsy failed to reveal the final pathology to determine further patient management. Biopsy pathology outcome, procedural details and technical success are listed in Table 2. Representative examples from four patients (patients #4, #5, #21, #24) are presented as Figures 1-4.
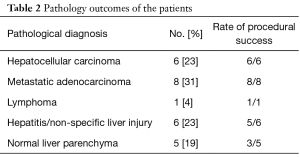
Full table
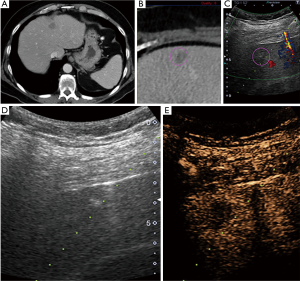
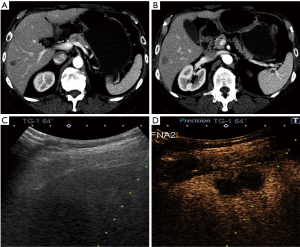
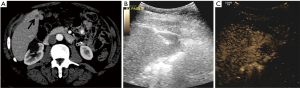
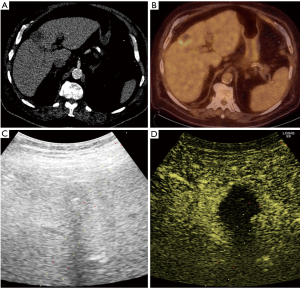
Discussion
CEUS for FLL involves intravenous injection of a microbubble contrast agent, in order to delineate the lesion from the surrounding liver parenchyma. In this study we administered Definity (Lantheus Medical Imaging, N. Billerica, MA, USA) and Lumason (Bracco Imaging, Monroe Township, NJ, USA). Definity is a second-generation US contrast agent consisting of lipid-stabilized perflutren microbubbles that act as a blood pool agent and true intravascular tracer, essentially not accumulating in the liver parenchyma. After activation of its component lipid microspheres by shaking the vial, Definity can be administered over approximately 10 to 30 seconds followed by 10 mL of IV saline flush, usually over 10 seconds. Lumason is a blood pool contrast agent composed of sulfur hexafluoride microbubbles with a phospholipid shell. During US imaging, a single 2.4 mL bolus of Lumason with 5 to 10 mL saline is injected and followed with IV saline push; a second Lumason 2.4 mL bolus may be given as clinically deemed necessary (21-23).
Multicenter studies have shown that ultrasound contrast agents have an excellent safety profile with incidence of serious adverse events ranging from 0.0086% to 0.031% (24-27). Furthermore, these contrast agents do not pose nephrotoxicity risks as they are fully excreted via the respiratory tract (28-30). This is particularly useful when considering that some patients with malignant FLL may require nephrotoxic iodine-based contrast agents at some point for liver-directed angiography based treatment.
Despite the cost and safety advantages of US as a guidance modality for percutaneous FLL biopsy, one of its drawbacks is the lack of consistency in accurate lesion visualization. Studies have shown deficiencies in B-mode US guided liver biopsies, as sensitivities for metastatic liver lesions were as low as 41% (9). Another study showed that the procedural success rate for liver biopsy after a single puncture pass was 23.4% with B-mode US, compared to 43.0% with CEUS (1) and that up to 30% of lesions were unable to be visualized on pre-treatment US for HCC (10). Therefore CEUS may be helpful to increase biopsy yield by superior visualization as shown in this study.
Several studies have suggested that administration of a contrast agent in patients with poorly visible hepatic lesions on US may improve diagnostic accuracy and reduce the number of puncture passes required to obtain a specimen for pathological diagnosis (1,19-21). These researches presented similar success rates in comparison to our results. A series by Schlottmann et al. demonstrated a success rate of 92% of CEUS guided liver biopsies (20). Yoon et al. showed a sensitivity of 88% for CEUS guided biopsies in the detection of liver malignancy (21). Sparchez et al. compared CEUS and conventional ultrasound guidance in percutaneous biopsies of liver tumors and showed an increased sensitivity of procedures performed under CEUS guidance, especially in large and poorly visible lesions (19). The technical success rate in our study was 88.5% and it needs to be considered that these were all selected patients which were not candidates of B-mode ultrasound guided biopsy because of lack of lesion visualization.
In this paper, we report our preliminary experience with CEUS guided biopsies of FLL that are poorly visualized on standard B-mode US based on a retrospective analysis. The decision to perform CEUS was made in the procedure suite based on clinical criteria. The alternative to a CEUS guided intervention under these circumstances, would have been a CT guided approach, therefore these cases can be considered challenging. Our method of patient selection mimicked a realistic clinical work flow, where only cases that failed under standard B-mode ultrasound were selected for CEUS guided procedures. This meant that only more complex cases were included in this study.
CEUS guided biopsy was successful in 23 out of 26 cases (88.5%) in obtaining a diagnostic specimen concordant with imaging and clinical follow-up. Meanwhile the unsuccessful cases (3/26, 11.5%) demonstrated no signs for liver malignancy on pathology, which was disconcordant with prior (CT/MR) imaging findings and follow-up, and therefore future re-biopsy was decided to be clinically necessary to determine further patient management.
This study has several limitations. The relatively small number of subjects was related to our institutional approach to select only challenging cases for CEUS guided biopsy as opposed to have every consecutive patient undergo CEUS guidance including cases which we were able to perform with regular B-mode ultrasound. This reflects a representative sample of lesions that truly required CEUS for better imaging guidance in a real-life clinical scenario. Another limitation is that there is inevitable subjectivity in evaluating FLL visualization on B-mode US due to lack of quantitative parameters. Furthermore, our study design was retrospective and thus we did not have a control group with which to compare study parameters. Prospective studies with a control group are warranted to confirm the results of this study and develop selection criteria for patients with FLL requiring tissue diagnosis.
In summary, this retrospective study demonstrated that in selected cases the addition of contrast enhancement to US can improve technical success rate and facilitate FLL guided biopsy in selected patients with poorly visualized FLL on standard B-mode US. In the past these selected patients would have undergone CT guided FLL biopsy. A larger prospective study is warranted to confirm these results and to develop criteria in which subjects CEUS guided FLL biopsy is advisable.
Acknowledgements
None.
Footnote
Conflicts of Interest: The results of the paper were partially presented as oral talk at the Society of Interventional Radiology 2017 Annual Meeting in Washington, DC in March 2017 and at the Digestive Disease Intervention Meeting 2016 in San Diego, CA in October 2016.
Ethical Statement: This study was approved by the institutional review board of University Hospitals Cleveland Medical Center (IRB No. 06-16-31). A waiver of informed consent was granted due to the retrospective fashion of the study.
References
- Spârchez Z, Radu P, Kacso G, et al. Prospective comparison between real time contrast enhanced and conventional ultrasound guidance in percutaneous biopsies of liver tumors. Med Ultrason 2015;17:456-63. [PubMed]
- Catalano O, Nunziata A, Lobianco R, et al. Real-time harmonic contrast material-specific US of focal liver lesions. Radiographics 2005;25:333-49. [Crossref] [PubMed]
- Cresswell AB, Welsh FK, Rees M. A diagnostic paradigm for resectable liver lesions: to biopsy or not to biopsy? HPB (Oxford) 2009;11:533-40. [Crossref] [PubMed]
- Huang GT, Sheu JC, Yang PM, et al. Ultrasound-guided cutting biopsy for the diagnosis of hepatocellular carcinoma--a study based on 420 patients. J Hepatol 1996;25:334-8. [Crossref] [PubMed]
- Durand F, Regimbeau JM, Belghiti J, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 2001;35:254-8. [Crossref] [PubMed]
- Fornari F, Filice C, Rapaccini GL, et al. Small (< or = 3 cm) hepatic lesions. Results of sonographically guided fine-needle biopsy in 385 patients. Dig Dis Sci 1994;39:2267-75. [Crossref] [PubMed]
- Vijayaraghavan GR, David S, Bermudez-Allende M, et al. Imaging-guided Parenchymal Liver Biopsy: How We Do It. J Clin Imaging Sci 2011;1:30. [Crossref] [PubMed]
- Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med 2001;344:495-500. [Crossref] [PubMed]
- Kinkel K, Lu Y, Both M, et al. Detection of hepatic metastases from cancers of the gastrointestinal tract by using noninvasive imaging methods (US, CT, MR Imaging, PET): a meta-analysis. Radiology 2002;224:748-56. [Crossref] [PubMed]
- Rhim H, Lee MH, Kim YS, et al. Planning sonography to assess the feasibility of percutaneous radiofrequency ablation of hepatocellular carcinomas. AJR Am J Roentgenol 2008;190:1324-30. [Crossref] [PubMed]
- Pang EH, Harris AC, Chang SD. Approach to the Solitary Liver Lesion: Imaging and When to Biopsy. Can Assoc Radiol J 2016;67:130-48. [Crossref] [PubMed]
- Albrecht T, Blomley MJ, Burns PN, et al. Improved detection of hepatic metastases with pulse-inversion US during the liver-specific phase of SHU 508A: multicenter study. Radiology 2003;227:361-70. [Crossref] [PubMed]
- Chami L, Lassau N, Malka D, et al. Benefits of contrast-enhanced sonography for the detection of liver lesions: comparison with histologic findings. AJR Am J Roentgenol 2008;190:683-90. [Crossref] [PubMed]
- Sporea I, Badea R, Popescu A, et al. Contrast-enhanced ultrasound (CEUS) for the evaluation of focal liver lesions - a prospective multicenter study of its usefulness in clinical practice. Ultraschall Med 2014;35:259-66. [Crossref] [PubMed]
- Celli N, Gaiani S, Piscaglia F, et al. Characterization of liver lesions by real-time contrast-enhanced ultrasonography. Eur J Gastroenterol Hepatol 2007;19:3-14. [Crossref] [PubMed]
- Nicolau C, Vilana R, Catalá V, et al. Importance of evaluating all vascular phases on contrast-enhanced sonography in the differentiation of benign from malignant focal liver lesions. AJR Am J Roentgenol 2006;186:158-67. [Crossref] [PubMed]
- Ricci P, Laghi A, Cantisani V, et al. Contrast-enhanced sonography with SonoVue: enhancement patterns of benign focal liver lesions and correlation with dynamic gadobenate dimeglumine-enhanced MRI. AJR Am J Roentgenol 2005;184:821-7. [Crossref] [PubMed]
- Website of the American College of Radiology. ACR news, May 27, 2016. Lumason© FDA reference ID: 3910678. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203684s001lbl.pdf
- Sparchez Z, Radu P, Zaharia T, et al. Usefulness of contrast enhanced ultrasound guidance in percutaneous biopsies of liver tumors. J Gastrointestin Liver Dis 2011;20:191-6. [PubMed]
- Schlottmann K, Klebl F, Zorger N, et al. Contrast-enhanced ultrasound allows for interventions of hepatic lesions which are invisible on convential B-mode. Z Gastroenterol 2004;42:303-10. [Crossref] [PubMed]
- Yoon SH, Lee KH, Kim SY, et al. Real-time contrast-enhanced ultrasound-guided biopsy of focal hepatic lesions not localised on B-mode ultrasound. Eur Radiol 2010;20:2047-56. [Crossref] [PubMed]
- Wu W, Chen MH, Yin SS, et al. The role of contrast-enhanced sonography of focal liver lesions before percutaneous biopsy. AJR Am J Roentgenol 2006;187:752-61. [Crossref] [PubMed]
- Maruyama H, Matsutani S, Saisho H, et al. Different behaviors of microbubbles in the liver: time-related quantitative analysis of two ultrasound contrast agents, Levovist and Definity. Ultrasound Med Biol 2004;30:1035-40. [Crossref] [PubMed]
- Porter TR, Abdelmoneim S, Belcik JT, et al. Guidelines for the cardiac sonographer in the performance of contrast echocardiography: a focused update from the American Society of Echocardiography. J Am Soc Echocardiogr 2014;27:797-810. [Crossref] [PubMed]
- Wei K, Mulvagh SL, Carson L, et al. The safety of deFinity and Optison for ultrasound image enhancement: a retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr 2008;21:1202-6. [Crossref] [PubMed]
- Herzog CA. Incidence of adverse events associated with use of perflutren contrast agents for echocardiography. JAMA 2008;299:2023-5. [Crossref] [PubMed]
- Main ML, Ryan AC, Davis TE, et al. Acute mortality in hospitalized patients undergoing echocardiography with and without an ultrasound contrast agent (multicenter registry results in 4,300,966 consecutive patients). Am J Cardiol 2008;102:1742-6. [Crossref] [PubMed]
- Ma F, Cang Y, Zhao B, et al. Contrast-enhanced ultrasound with SonoVue could accurately assess the renal microvascular perfusion in diabetic kidney damage. Nephrol Dial Transplant 2012;27:2891-8. [Crossref] [PubMed]
- Hutter JC, Luu HM, Mehlhaff PM, et al. Physiologically based pharmacokinetic model for fluorocarbon elimination after the administration of an octafluoropropane-albumin microsphere sonographic contrast agent. J Ultrasound Med 1999;18:1-11. [Crossref] [PubMed]
- Definity (perflutren) Injection Label. Food and Drug Administration. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021064s011lbl.pdf


