Clinicopathological features and survival outcomes of primary signet ring cell and mucinous adenocarcinoma of colon: retrospective analysis of VACCR database
Introduction
Signet ring cell carcinoma (SRCC) of colon and mucinous adenocarcinoma (MCC) of colon are rare histologic subtypes of adenocarcinoma of colon accounting for approximately 0.5-1 percent and 15-20 percent of all adenocarcinomas of colon respectively (1). Signet ring cell cancers are most commonly seen in the stomach (95%) and occasionally found in colon, rectum, ovary, peritoneum and gallbladder (2). It is characterized by specific morphologic appearance of abundant intracytoplasmic mucin pushing nucleus to the periphery giving it a signet ring cell appearance. SRCC is similar to MCC in possessing abundant mucin. The World Health Organization classification of tumors has a specific criteria for diagnosis of these sub types--SRCC is defined as presence of more than 50 percent of signet cells and MCC is defined as presence of more than 50 percent of mucin component (3).
Previous studies have shown that SRCC often presents at young age, in advanced stage, with more peritoneal involvement and has poor prognosis (4,5). However, majority of these studies are single institution based including small number of patients. Because of the rarity of the disease, clinico-pathological features and prognosis has not been well understood and there have been very few studies comparing SRCC with MCC and non-mucinous adenocarcinoma (NMCC) of colon. Hence we conducted a retrospective study on the large nationwide veteran population to understand the clinicopathological features and the survival outcomes of SRCC, MCC and NMCC.
Methods
Data source
The study was approved by the local Institutional Review Board. Data for this study was obtained by accessing the Veteran’s Affairs Central Cancer Registry (VACCR) database. VACCR is a population-based registry sponsored by the Veteran’s Affairs Healthcare system that contains information from patients diagnosed and/or treated at all 143 Veterans Affairs (VA) medical centers. Each case report adheres to the standards established by the American College of Surgeons’ Commission on Cancer Facility Oncology Registry Data Standards for data collection and definitions and must pass North American Association of Central Cancer Registry electronic quality assurance edits before being merged/consolidated into the master database.
Study population
A total of 36,260 Veteran’s diagnosed with colon cancer between January 1995 and December 2008 were identified from the VACCR database. Of which 26,669 were NMCC patients, 2,443 were MCC patients, and 206 were SRCC patients and 6,942 were other histology’s. Colon cancer cases associated with other histology’s, inflammatory bowel disease and familial polyposis syndromes were excluded from this study.
Patient characteristics and survival data
The variables recorded were patient age, gender, ethnicity, clinical stage, pathological stage, histology, grade, location of tumor, number of regional lymph nodes retrieved, number of positive lymph nodes, date of diagnosis, CEA, sites of metastases, type of surgery, surgical margins and treatment. Tumor histology was classified by using the International Classification of Histology (ICD-O-3) into NMCC [8,140, 8,243-8,245, 8,210, 8,211], MCC [8,470, 8,472, 8,480, and 8,481] and SRCC [8,490]. Tumor locations were divided into cecum, appendix, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon and overlapping tumors (NOS) based on the SEER Program Coding and Staging Manual 2007. Tumor stage was based on TNM staging system and American Joint Committee on Cancer, AJCC Cancer Staging Manual (6th edition, 2002). Tumor grade was further classified as well differentiated, moderately differentiated, poorly differentiated, undifferentiated or anaplastic tumors. Overall survival was calculated for patients not alive as total number of months from date of diagnosis to date of last contact and for those alive as total number of months from date of diagnosis to July 30, 2009 i.e., when the database was last updated.
Statistical analysis
Quantitative variables such as age and number of lymph nodes were summarized as mean and standard deviation. One-way ANOVA model was fitted to a continuous variable to examine if means of several groups are all equal. Univariate logistic regression analysis was performed to determine the factors significantly associated with various histologies. Chi-square analysis was used to compare differences between NMCC, MCC and SRCC. For all statistical tests, significance level is set at 0.05. Statistical analysis was carried out with SAS 9.2 (SAS Institute Inc., Cary NC).
Results
Demographics
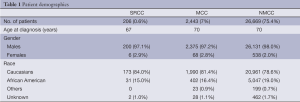
Of 36,260 colon cancer patients, 26,669 (73.5 percent) were NMCC, 2,443 (7 percent) were MCC and 206 (0.6 percent) were SRCC patients. Median age at diagnosis of SRCC was 67 years as compared to 70 years for both MCC and NMCC. Study patients were mainly males and caucasians. There were no significant gender differences noted among the three histological subtypes. However, African Americans were found to have less SRCC and MCC incidence as compared to NMCC (15%, 16.4% and 19%, respectively). Detailed demographic data is shown in Table 1.
Full table
Location of tumor
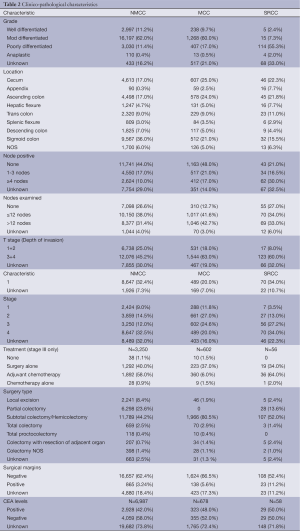
SRCC and MCC were more common on the right side of the colon involving cecum and ascending colon (59.5%, 56.5% and 39%) while NMCC was most commonly found on the left side of the colon involving the sigmoid colon (23%, 29.5% and 46%). The individual distribution of SRCC, MCC and NMCC across various portions of the colon was shown in Table 2.
Full table
Grade
The percentage of SRCC, MCC and NMCC patients significantly varied across the grade distribution with SRCC often presented as high-grade tumors (poorly differentiated or undifferentiated: SRCC, 55.3%; MCC, 17%; NMCC, 11.4%) while MCC and NMCC presented as moderately differentiated tumors (SRCC, 7.3%; MCC, 60%; NMCC, 62%).
Tumor invasion
The majority of SRCC and MCC patients had diffuse colonic wall invasion at the time of presentation often involving sub serosa and serosal layers as represented by their T stage. Pathological T-stages at presentation among SRCC, MCC and NMCC were as follows: T3 + T4 were 60%, 63% and 45.2%; T1 + T2 were 8%, 18% and 25%, respectively.
Nodal involvement
The majority of SRCC had nodal involvement at the time of presentation unlike MCC and NMCC. The nodal status at the time of presentation among three histological subtypes is detailed in Table 2. Percentage of node negative disease among SRCC, MCC and NMCC was 21%, 48% and 44% respectively. We also noted no significant differences in number of lymph nodes retrieved among SRCC, MCC and NMCC (<12 nodes retrieved was 34%, 42% and 38%; >12 nodes examined was 33%, 43% and 31% respectively).
AJCC stage
In terms of stage, SRCC often presents as advanced stage (stage 3+4: SRCC, 61.2%; MCC, 44.6%; NMCC, 44.5%) while MCC and NMCC were early stage at presentation (stage 1+2: SRCC, 16.5%; MCC, 38.8%; NMCC, 23.5%). Percentages of unknowns: SRCC, 22.3%; MCC, 16%; NMCC, 32%.
Carcinoembryonic antigen (CEA) levels
CEA levels were not available for most of the patients (SRCC, 71.8%; MCC, 72.4%; NMCC, 73.8%). However, from the limited available data, majority of the SRCC and MCC patients had high CEA levels as compared to NMCC (SRCC, 50%; MCC, 48%; NMCC, 42%).
Treatment
A majority of stage III SRCC patients received adjuvant chemotherapy compared to MCC and NMCC. As treatment is mainly stage specific we included only stage III patients while analyzing for adjuvant chemotherapy (64%, 60% and 58%).
Type of surgery and surgical margins
The number of patients who underwent subtotal colectomy and/or hemicolectomy were 107 (52%), 1,966 (80.5%) and 11,789 (44.2%) in SRCC, MCC and NMCC groups respectively. The surgical margins were positive in 11.2% of SRCC patients, 5.6% of MCC patients and 3.2% of NMCC patients.
Survival analysis
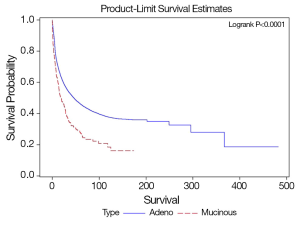
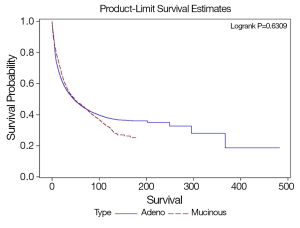
SRCC has worse overall survival compared to MCC and NMCC. The median survival of SRCC as compared to NMCC was 18.6 and 46 months respectively (P<0.0001), and MCC as compared to NMCC was 47.8 and 46 months respectively (P=0.63). The stage specific average five-year survivals were shown in Table 3. In our study early stage SRCC and MCC had better five-year survival compared to NMCC while advanced stage SRCC and MCC had worse survival compared to NMCC (Stage I: SRCC, 100%; MCC, 61%; NMCC, 41%; P<0.0001. Stage II: SRCC, 42%; MCC, 58%; NMCC, 32%; P<0.0001. Stage III: SRCC, 19%; MCC, 41%; NMCC, 47%; P=0.0002. Stage IV: SRCC, 1.5%; MCC, 7%; NMCC, 31%; P<0.0001). The small number of patients with early stage SRCC could have affected the survival. Stage specific and overall survival of SRCC, MCC and NMCC are shown in Table 3, Figures 1,2.
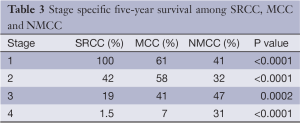
Full table
Discussion
SRCC and MCC are well recognized subtypes of colorectal carcinoma but are uncommon in occurrence. The frequencies of SRCC and MCC in our study are 0.6% and 7% respectively and our study is one of the largest series reported so far. These incidence rates are similar to that mentioned in other studies (1,4,6) with an incidence rate of nearly 1% for SRCC and 5-15% for MCC.
SRCC occurs at younger age compared to MCC and NMCC. Median age of diagnosis is 67 years in our study, which is higher than that mentioned in few single institution studies (50.8 years) (7). However it is very similar to those mentioned in other large population based studies (4). The difference in age at presentation is likely due to the bias associated with single institution studies. In our series we found SRCC patients to have significantly higher incidence of poorly differentiated tumors, larger tumor size, proximal colonic tumor location and higher CEA levels. In addition, we found both mucinous and signet-ring cell type tumors were more likely to have lymph node involvement and organ infiltration. These findings are consistent with prior studies (5,8).
SRCC has poor survival rates compared to MCC and NMCC. The survival rates of MCC are similar compared to NMCC, which is consistent with few other studies (4,9,10), especially after adjusting for stage (11). SRCC’s poor outcomes might be related to higher tumor stage and grade, propensity for nodal as well as peritoneal involvement however the reasons for these features are not well understood. SRCC is considered as a tumor arising in flat colonic mucosa and not following the adenoma-carcinoma sequence (12). This probably explains the reason for fewer patients being diagnosed in early stages. This also has implications in colon cancer screening with colonoscopy where these tumors are not easily visualized. A DNA based stool testing might overcome this issue in future (13).
Molecular mechanisms underlying the pathogenesis of SRCC have been evaluated to better understand the aggressive nature of this disease. Several candidate genes based on gene expression analysis have been studied however the exact molecular mechanisms are not well understood. Colon cancers with high-frequency microsatellite instability (MSI) have in general better survival outcomes. However, both SRCC and NMCC, inspite of increased rates of high-frequency MSI the prognosis is poor suggesting varied carcinogenesis in these tumors (14,15). SRCC are also found to have low rates of K-ras mutations and higher B-raf mutations compared to NMCC (16,17). B-raf mutations are considered an independent poor prognostic factor in colorectal cancer (18,19). Park et al. has shown higher expression of mucin regulating genes such as HATH1, MUC2 and SOX215 and Sentani et al. also reported high expression of MUC2, MUC5, Reg IV and Claudin 18 in SRCC (20,21). Overexpression of these genes leads to large amounts of intracellular mucin production, eventually forming clusters of cells, which disrupt the E-cadherin/β-catenin complex and cell-cell adhesions facilitating diffuse spread of the tumor. Ogni et al. has proposed that higher frequency of the CpG island methylator phenotype (CIMP) in SRCC leads to aberrant hyper methylation and reduced expression of E-cadherin (17). Others have hypothesized that the mucopolysaccharide of colloid-type carcinomas jams discrimination of host immunocytes from tumor cells, thus these colloid-secreting carcinomas easily invade peri-intestinal tissue resulting in infiltration into lymphatic vessels and nodes (12).
The main limitation of our study is lack of central pathology review. Retrospective nature, predominant male population and lack of information regarding patient preferences, performance status and physician biases are other limitations of the study. Despite these limitations, our study represents one of the largest retrospective studies of SRCC of colon.
Conclusions
In conclusion, mucinous and SRCCs have unique clinicopathological features and are more aggressive in biologic behavior than the common NMCC. SRCC is a poor individual prognostic factor. Because of the rarity of the tumor, prospective multi-institute studies with a special focus on gene expression, may lead to development of targeted therapies and improved survival outcomes of these patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Almagro UA. Primary signet-ring carcinoma of the colon. Cancer 1983;52:1453-7. [PubMed]
- Tung SY, Wu CS, Chen PC. Primary signet ring cell carcinoma of colorectum: an age- and sex-matched controlled study. Am J Gastroenterol 1996;91:2195-9. [PubMed]
- Hamilton SR, Rubio CA, Vogelstein B, et al. Carcinoma of the colon and rectum. In: Hamilton SR, Aaltonen LA. eds. World Health Organization classification of tumours. Tumours of the digestive system. Lyon, France: IARC Press, 2000:101-19.
- Kang H, O’Connell JB, Maggard MA, et al. A 10-year outcomes evaluation of mucinous and signet-ring cell carcinoma of the colon and rectum. Dis Colon Rectum 2005;48:1161-8. [PubMed]
- Sasaki S, Masaki T, Umetani N, et al. Characteristics in primary signet-ring cell carcinoma of the colorectum, from clinicopathological observations. Jpn J Clin Oncol 1998;28:202-6. [PubMed]
- Anthony T, George R, Rodriguez-Bigas M, et al. Primary signet-ring cell carcinoma of the colon and rectum. Ann Surg Oncol 1996;3:344-8. [PubMed]
- Sung CO, Seo JW, Kim KM, et al. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod Pathol 2008;21:1533-41. [PubMed]
- Lee WS, Chun HK, Lee WY, et al. Treatment outcomes in patients with signet ring cell carcinoma of the colorectum. Am J Surg 2007;194:294-8. [PubMed]
- Du W, Mah JT, Lee J, Sankila R, et al. Incidence and survival of mucinous adenocarcinoma of the colorectum: a population-based study from an Asian country. Dis Colon Rectum 2004;47:78-85. [PubMed]
- Wu CS, Tung SY, Chen PC, et al. Clinicopathological study of colorectal mucinous carcinoma in Taiwan: a multivariate analysis. J Gastroenterol Hepatol 1996;11:77-81. [PubMed]
- Symonds DA, Vickery AL. Mucinous carcinoma of the colon and rectum. Cancer 1976;37:1891-900. [PubMed]
- Ponz de Leon M, Di Gregorio C. Pathology of colorectal cancer. Dig Liver Dis 2001;33:372-88. [PubMed]
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 2004;351:2704-14. [PubMed]
- Kakar S, Smyrk TC. Signet ring cell carcinoma of the colorectum: correlations between microsatellite instability, clinicopathologic features and survival. Mod Pathol 2005;18:244-9. [PubMed]
- Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69-77. [PubMed]
- Song GA, Deng G, Bell I, et al. Mucinous carcinomas of the colorectum have distinct molecular genetic characteristics. Int J Oncol 2005;26:745-50. [PubMed]
- Ogino S, Brahmandam M, Cantor M, et al. Distinct molecular features of colorectal carcinoma with signet ring cell component and colorectal carcinoma with mucinous component. Mod Pathol 2006;19:59-68. [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [PubMed]
- Safaee Ardekani G, Jafarnejad SM, Tan L, et al. The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS One 2012;7:e47054. [PubMed]
- Sentani K, Oue N, Tashiro T, et al. Immunohistochemical staining of Reg IV and claudin-18 is useful in the diagnosis of gastrointestinal signet ring cell carcinoma. Am J Surg Pathol 2008;32:1182-9. [PubMed]
- Park SY, Lee HS, Choe G, et al. Clinicopathological characteristics, microsatellite instability, and expression of mucin core proteins and p53 in colorectal mucinous adenocarcinomas in relation to location. Virchows Arch 2006;449:40-7. [PubMed]