Radiation dose in neoadjuvant chemoradiation therapy for esophageal cancer: patterns of care and outcomes from the National Cancer Data Base
Introduction
Esophageal cancer (EC) is a major cause of both cancer-related mortality worldwide, with an estimated 400,000 annual deaths attributable to this disease (1). The current standard of care is neoadjuvant chemoradiation (CRT) followed by definitive surgery, based on the landmark publication of the CROSS trial in 2012, which has provided the most definitive evidence for trimodality therapy in locally advanced EC (2,3). The CROSS protocol consisted of preoperative carboplatin/paclitaxel administered concurrently with radiation therapy (RT) to a dose of 41.4 Gy. This dose was somewhat unconventional in light of many prospective trials that utilized 45 Gy (4-6). However, prospective trials have, in fact, utilized total doses as low as 35–37 Gy as part of preoperative multimodality management, with satisfactory results (7,8).
Lower dose (LD) CRT has the theoretical (albeit largely unproven) advantage of providing “cleaner” dissection planes during esophagectomy as well as reducing risks of postoperative complications and mortality. However, radiation oncologists may be hesitant to utilize LDs in clinical practice for multiple reasons. First, 45 Gy is often thought to be the “minimal” dose required to sterilize microscopic disease (especially in areas where surgical dissection may not take place, such as elective nodal treatment) based on radiotherapeutic principles (9). Second, higher doses (HDs) may be associated with higher rates of margin-free resection. Third, if a patient does not end up undergoing resection for various reasons, a dose of 50–50.4 Gy is the recommended dose for definitive CRT (2), and treatment with this dose entirely up front prevents a potential treatment gap. As such, national guidelines endorse a wide dose range of 41.4 to 50.4 Gy in the neoadjuvant setting (2).
This study is the first to date evaluating national practice patterns and outcomes of EC patients undergoing neoadjuvant CRT using LD [40–41.4 Gy (3,10)] versus HD [50–50.4 Gy (2)] RT. We specifically sought to assess trends and patterns of care in the utilization of LD versus HD CRT, evaluate whether overall survival (OS) was different between these cohorts, and whether postoperative events were affected by RT dosage changes.
Methods
This investigation analyzed the National Cancer Data Base (NCDB), which is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society, which consists of de-identified information regarding tumor characteristics, patient demographics, and patient survival for approximately 70% of the US population (11-14). The NCDB contains information not included in the Surveillance, Epidemiology, and End Results database, including details regarding use of systemic therapy and radiation dose. The data used in the study were derived from a de-identified NCDB file. The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data by the investigators. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
The most recently released NCDB dataset corresponded to the years 2004–2013. Inclusion criteria for this study involved patients age >18 with newly-diagnosed cT1b-T4a N0/N+ M0 EC comprising histologic codes of adenocarcinoma [International Classification of Disease for Oncology (ICD-O-3) codes 8140, 8141, 8143, 8144, 8145, 8147, 8255, 8260, 8310, 8340, 8480, 8481] or squamous cell carcinoma (ICD-O-3 codes 8052, 8053, 8070, 8071, 8072, 8073, 8074, 8075, 8076, 8078, 8083, 8084, 8560). For inclusion, patients required histological diagnostic confirmation, receipt of neoadjuvant CRT followed by partial or complete esophagectomy {surgical procedure of the primary site codes [30, 40, 50–55, 80]}. Since the purpose of the study was to compare the effect of dose escalated neoadjuvant RT, we only included patients receiving either 40–41.4 Gy (3,10) which was classified as low dose radiation (LD) or 50–50.4 Gy (2) which was classified as high dose radiation (HD). The use of concurrent therapy was defined as receipt of chemotherapy within 15 days of radiation.
Information collected on each patient broadly included demographic data, comorbidity information, clinicopathologic tumor parameters, and treatment facility characteristics. All statistical tests were two-sided, with a threshold of P<0.05 for statistical significance, and were performed using STATA (version 14, College Station, TX, USA). Fisher’s exact or χ2 test analyzed categorical proportions between groups in the non-parametric and parametric settings, respectively. Multivariable logistic regression modeling was utilized to determine characteristics that were predictive for receipt of LD RT. The Kaplan-Meier method was used for survival analysis, and comparisons between the LD and HD groups were performed with the log-rank test. OS was defined as the interval between the date of diagnosis and the date of death or last contact. Univariate analysis was performed to determine which factors were associated with OS, and subsequently Cox multivariate analysis was performed including variables that were either significant or showed a strong trend to statistical significance on univariate analysis.
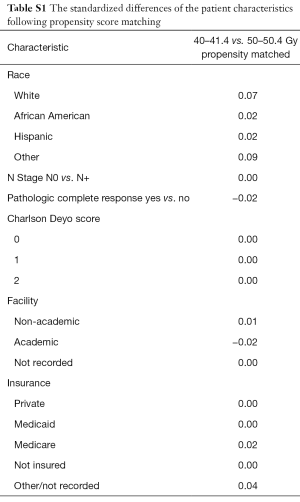
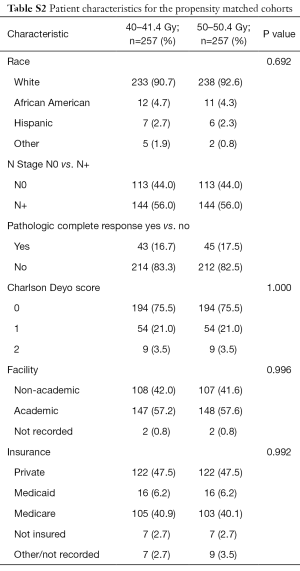
To account for indication bias, propensity score matching was used to compare patients treated with each of the RT dose/fractionation schemes. Propensity matching is a method that creates quasi case/control pairs using a retrospective cohort in an effort to account for the recorded and unrecorded confounding variables (15-17). Propensity scores were calculated by use of a multivariable logistic regression model with the dependent variable being receipt of treatment with LD vs. treatment with HD and the independent variables being those that were statistically significant for correlation with OS on multivariate analysis. Patients were matched 1:1 without replacement to avoid potential bias from many-to-one matching. In order to ensure balance, a caliper of 0.05 was selected. Standardized differences were assessed in order to ensure balance between each of the variables included in calculating the propensity score the matched cohorts with a value <0.1 signifying an inconsequential imbalance (18) (Table S1). Pearson’s χ2 test was subsequently performed between the matched cohorts to confirm balance amongst the variable (Table S2). Survival rates were then compared between the two matched groups with the log-rank test.
Full table
Full table
Results
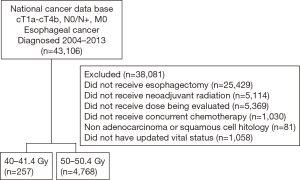
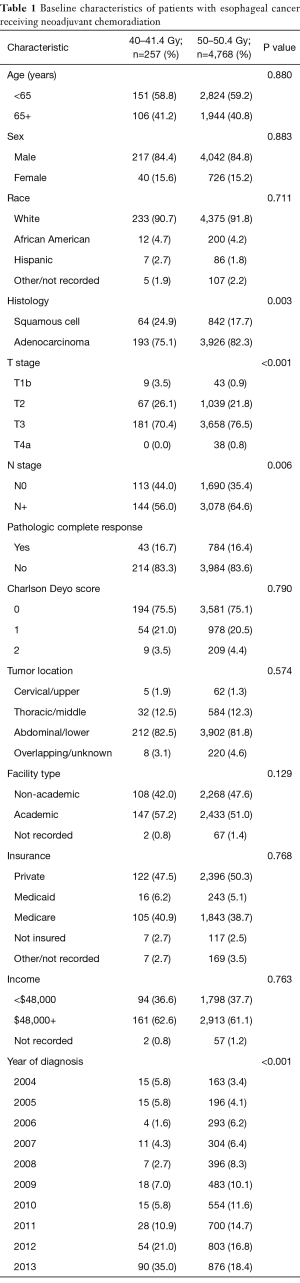
A complete flow diagram of patient selection is provided in Figure 1; 5,025 patients met study criteria. Of these, 257 (5%) were treated with LD RT, and 4,768 (95%) HD RT. Table 1 displays clinical characteristics of the analyzed patients. Of note, most patients had adenocarcinomas located in the distal esophagus and locally advanced disease.
Full table
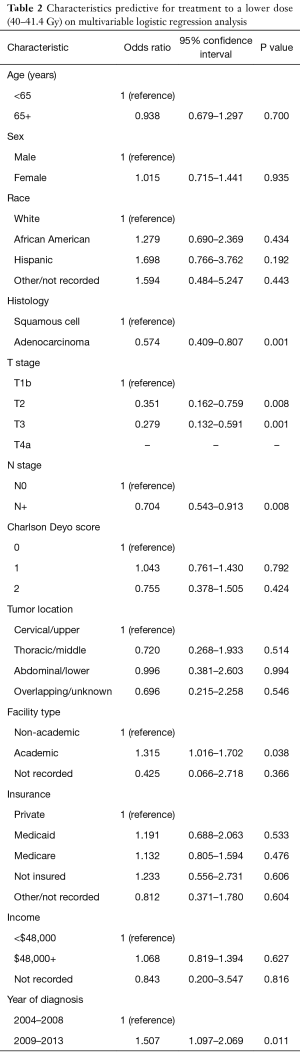
Multivariable logistic regression analysis was performed to evaluate factors independently associated with undergoing LD therapy (Table 2). LDs were more likely delivered at academic centers (P=0.038), in more recent years (2009–2013, P=0.011), and to squamous cell carcinomas (P=0.001). HD RT tended to be administered in patients with higher T classification as well as node-positive disease (P<0.05 for all).
Full table
Median follow-up was 25.9 months [interquartile range (IQR), 14.5–44.3 months]. Kaplan-Meier estimates of OS between groups are illustrated in Figure 2A. The median OS in the LD group was 39.0 and 35.6 months in the HD cohort (P=0.071).

However, because both groups were imbalanced in terms of several variables, propensity matching was performed in order to evaluate OS between more balanced populations. When examining OS between both propensity matched cohorts (Figure 2B), there were no OS differences between cohorts (39.0 vs. 42.9 months, P=0.812).
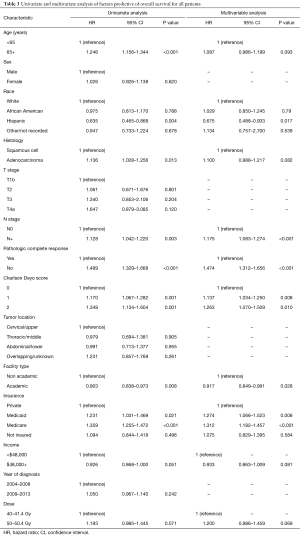
Multivariate Cox proportional hazards modeling examining independent predictors of OS is displayed in Table 3. There were several factors associated with poorer OS: node-positive disease, lack of pathologic complete response (pCR), presence of comorbidities, Medicare/Medicaid insurance, and treatment at a community facility (P<0.05 for all). Of note, dose did not independently correlate with OS [hazard ratio (HR), 1.20; 95% confidence interval (CI), 0.986–1.459; P=0.069].
Full table
Lastly, the NCDB tabulates postoperative mortality, length of hospital stay, and readmission rates, which were analyzed between groups. Respective 30-day readmission rates in the LD and HD groups were 7.4% and 5.8% (P=0.182). The 30- and 90-day mortality rates were 1.6% vs. 3.2% (P=0.314) and 4.3% vs. 7.7% (P=0.007). The mean length of postoperative hospital stay was 12.8 days (95% CI: 11.4–14.3 days) vs. 13.2 days (95% CI: 12.8–13.5 days), respectively (P=0.665).
Discussion
Although the value of trimodality therapy in EC has now been more clearly defined, there remains a degree of controversy regarding the more practical point of RT dosing when delivered preoperatively (19). There are numerous findings and reflections from this analysis of a contemporary national database, the largest of its kind to date. In the United States, LD RT is underutilized as compared to HD RT, but does show changes based on treatment period, treatment facility, and histology. There were no differences between cohorts in terms of survival or most postoperative outcomes, which persisted after propensity matching and multivariate adjustment. These findings have notable implications for the practical aspects of multimodal management of EC.
Although this investigation of a contemporary database excluded patients treated to a commonly-utilized dose of 45 Gy (in light of these results, it is unlikely to find differences in endpoints between regimens differing by just 2–3 fractions), it is still feasible to conclude that even LD regimens in multiple phase III trials (3,10) are clearly underutilized in the United States as compared to HD RT. However, time period (2009–2013 vs. 2004–2008) was independently associated with greater use of LD RT. Because the CROSS trial was published in 2012 and the final year of NCDB data collection included in the present analysis was in 2013, most recent years are unable to be analyzed. Nevertheless, these results are consistent with an unpublished survey of 274 US radiotherapy providers, revealing that 50.4 Gy was the most preferred dose in the neoadjuvant setting (20). Rationales for such included the ability to achieve margin-free resection at the cost of increased adverse events. However, whereas 86% of respondents felt that HDs would lead to increased pCR rates, this was not found in our data, which revealed a pCR rate of about 16% in both the LD and HD cohorts.
It is readily acknowledged that both groups were imbalanced prior to propensity matching, given the intuitiveness of delivering more “aggressive” doses to “higher-risk” disease (21), as exemplified by the more advanced T classification and nodal positivity in the HD population. The NCDB also cannot account for tumor volume, which does not necessarily equate to T classification. Nevertheless, these findings are readily explained by the trends toward inferior OS in the HD group (P=0.072) along with the association on Cox multivariate analysis (P=0.069), whereas the propensity matched analysis resulted in no significant trends. Nevertheless, based on the retrospective shortcomings of this study, our results should not necessarily be interpreted as no differences between the HD and LD arms; rather, we posit that there is no evidence supporting routine delivery of HD RT in all cases. On the basis of this study, it may be reasonable to deliver LD RT in squamous cell histology [given that nearly half develop a pCR to LD RT (3)] and/or lower-volume disease. Alternatively, LD RT may also have utility in patients with borderline performance status and/or at risk of adverse or postoperative events (discussed further below). Nevertheless, each patient should be evaluated while taking into account both tumor specific characteristics as well as the patient’s underlying comorbidities before a decision on dose is made.
In addition to patients with squamous cell carcinoma, academic centers were also more likely to deliver LD RT, which could relate to greater use of evidence-based medicine in such institutions. However, the independent association between treatment at an academic facility and OS on Cox multivariate analysis has far-reaching implications on patient counseling and management by both oncologists and referring providers. These findings are in concord with data from other neoplasms demonstrating improved outcomes at academic and/or high-volume facilities (22). There are several potential reasons for this, not limited to greater multimodality coordination, streamlined diagnostic processes, technical expertise of a major surgical procedure, ancillary support staff for close clinical monitoring, and potentially the availability of salvage therapies (or clinical trials). Nevertheless, this finding may impact any case of locally advanced EC and could warrant revisions in patterns of patient education.
The findings of statistically insignificant differences in 30-day postoperative mortality, length of hospital stay, and 30-day readmission rates are especially important for surgical providers. However, this study noted slightly worse 90-day mortality in the HD cohort. In the absence of statistically significant 30-day mortality figures, there are many possible explanations for this observation. Because the NCDB does not give causes of death at these time points, it is first unclear whether this mortality figure is specifically related to surgery. However, if these values are ascribed to surgery, it is possible that patients in the HD (having higher T stage and node positive disease) required more extensive surgeries and hence a greater likelihood of complications. This is consistent with a retrospective single-institution report of HD therapy being associated with more acute adverse effects and potentially suboptimal surgical conditions (23). Nevertheless, in light of studies demonstrating that advanced RT techniques may decrease adverse and postoperative events, further work must assess whether a decrease in dose has additive effects as well (24-28).
There are several shortcomings of this investigation. First, issues regarding retrospective selection biases and imbalances between cohorts have been discussed above. Second, the NCDB’s coding of RT dose (specific treatment volumes are also not reported) may be incongruous between reporting institutions, because there is likely little standardization between receiving 45 Gy plus a 5.4 Gy boost as compared to 50.4 Gy with no boost. Third, the NCDB does not keep track of several noteworthy variables, such as reasons for a particular treatment, elective nodal coverage, premature cessation of therapy, and salvage treatments. Although receipt of chemotherapy is recorded, specific agents are not mentioned. This is important in light of conflicting single-institutional data claiming higher OS in patients receiving cisplatin/5-fluorouracil (29) versus carboplatin/paclitaxel (30). Although the NCDB has a record of surgical margins, this information is very frequently missing; it also does not record other endpoints such as tolerance of therapy (including specific postoperative complications or toxicities in general), cancer-specific survival, and local/regional control. Lastly, the inclusion of T1-2 N0 patients (similar to the CROSS study) may bias towards no dose-related differences between groups, as patients most likely to benefit from dose-escalated therapy are more advanced cases. Nevertheless, the caveats herein do not obviate the need for further investigation to corroborate these conclusions.
Conclusions
Although the use of LD (40–41.4 Gy) RT as part of neoadjuvant CRT is underutilized, it is increasing in recent years, and is utilized more at academic centers and for squamous cell carcinoma. There were no differences in survival (before or after propensity matching), 30-day readmission/mortality, or length of postoperative hospital stay between LD and HD (50–50.4 Gy) therapy, nor did dose independently predict for survival. Trimodality therapy at academic institutions is independently associated with higher survival.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation.
References
- Siegel R, Ma J, Zhou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Esophageal and Esophagogastric Junction Cancers. Version 1. 2017. Available online: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#esophageal. Accessed July 14, 2017.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Urba SG, Orringer MB, Turrisi A, et al. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol 2001;19:305-13. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: Final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014;32:2416-22. [Crossref] [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: A randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-7. [Crossref] [PubMed]
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6th edition. Philadelphia, PA: Lippincott, Williams, and Wilkins, 2012.
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Bilimoria KY, Stewart A, Winchester D, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- Haque W, Verma V, Fakhreddine M, et al. Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: Analysis of the National Cancer Data Base. Gynecol Oncol 2017;144:28-33. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Patterns of care and outcomes of multi-agent versus single-agent chemotherapy as part of multimodal management of low grade glioma. J Neurooncol 2017;133:369-75. [Crossref] [PubMed]
- Moreno AC, Verma V, Hofstetter WL, et al. Patterns of Care and Treatment Outcomes of Elderly Patients With Stage I Esophageal Cancer: Analysis of the National Cancer Data Base. J Thorac Oncol 2017;12:1152-60. [Crossref] [PubMed]
- Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to- one matching on the propensity score. Am J Epidemiol 2010;172:1092-7. [Crossref] [PubMed]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:15. [Crossref]
- Austin PC, Gootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: A Monte Carlo study. Stat Med 2007;26:734-53. [Crossref] [PubMed]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083-107. [Crossref] [PubMed]
- Post CM, Haefner MF, Lin C, et al. Neoadjuvant versus definitive chemoradiation for locally advanced esophageal squamous cell carcinoma. Transl Cancer Res 2017;6:S625-8. [Crossref]
- Elliott DA, Nabavizadeh N, Kusano AS, et al. Locally Advanced Esophageal Chemoradiation Therapy Practice Patterns: Results From a National Survey of ASTRO Members. Int J Radiat Oncol Biol Phys 2015;93:S219. [Crossref]
- Verma V, McMillan MT, Grover S, et al. Stereotactic body radiation therapy and the influence of chemotherapy on overall survival for large (≥5 centimeter) non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2017;97:146-54. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Definitive chemoradiation at high volume facilities is associated with improved survival in glioblastoma. J Neurooncol 2017;135:173-81. [Crossref] [PubMed]
- Nabavizadeh N, Shukla R, Elliott DA, et al. Preoperative carboplatin and paclitaxel-based chemoradiotherapy for esophageal carcinoma: results of a modified CROSS regimen utilizing radiation doses greater than 41.4 Gy. Dis Esophagus 2016;29:614-20. [Crossref] [PubMed]
- Chuong MD, Hallemeier CL, Jabbour SK, et al. Improving Outcomes for Esophageal Cancer using Proton Beam Therapy. Int J Radiat Oncol Biol Phys 2016;95:488-97. [Crossref] [PubMed]
- Gharzai L, Verma V, Denniston KA, et al. Radiation Therapy and Cardiac Death in Long-Term Survivors of Esophageal Cancer: An Analysis of the Surveillance, Epidemiology, and End Result Database. PLoS One 2016;11:e0158916. [Crossref] [PubMed]
- Verma V, Lin SH, Simone CB 2nd, et al. Clinical outcomes and toxicities of proton radiotherapy for gastrointestinal neoplasms: a systematic review. J Gastrointest Oncol 2016;7:644-64. [Crossref] [PubMed]
- Verma V, Moreno AC, Lin SH. Advances in Radiotherapy Management of Esophageal Cancer. J Clin Med 2016;5:E91. [Crossref] [PubMed]
- Lin SH, Merrell KW, Shen J, et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother Oncol 2017;123:376-81. [Crossref] [PubMed]
- Haisley KR, Hart KD, Nabavizadeh N, et al. Neoadjuvant chemoradiotherapy with concurrent cisplatin/5-fluorouracil is associated with increased pathologic complete response and improved survival compared to carboplatin/paclitaxel in patients with locally advanced esophageal cancer. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- Sanford NN, Catalano PJ, Enzinger PC, et al. A retrospective comparison of neoadjuvant chemoradiotherapy regimens for locally advanced esophageal cancer. Dis Esophagus 2017;30:1-8. [Crossref] [PubMed]