Tumor response evaluation after neoadjuvant chemotherapy in locally advanced gastric adenocarcinoma: a prospective, multi-center cohort study
Introduction
Although many surgical and medical strategies have been proposed in the treatment of gastric adenocarcinoma, this tumor remains a therapeutic challenge. Nowadays gastric cancer is the fourth neoplasia in the world, even though its overall incidence has been decreasing considerably for the last 20 years (1). Presentation at last pathological stages and high risk of metastatic lesions at diagnosis make this tumor one of the most common cause of cancer death worldwide. Radical surgery is the only therapy with curative intent for locally advanced gastric cancer; however the 5-year survival rate for completely resected tumors is only 25–35% on average (2). The reason for these poor outcomes lays in the high risk of local relapse after surgery, probably due to the early lymph node invasion, which is a distinguishing feature of gastric adenocarcinoma. In order to improve the loco-regional control of this tumor, many studies have been established combining surgery with chemotherapy, radiotherapy or chemo-radiotherapy, both in the adjuvant or the neoadjuvant setting. Although treatment modalities differ significantly around the world, perioperative chemotherapy, which consists in giving half cycles of chemotherapy before and half cycles after surgery, is the principally adopted in Europe. Two randomized trials, the MAGIC study and the FNLCLCC/FFCD study, have shown that perioperative chemotherapy does significantly improve the overall survival (OS) and the disease free survival (DFS) of patients with gastric adenocarcinoma and esophagogastric junction adenocarcinoma (3,4). However, the effectiveness of such an approach applied to locally advanced gastric adenocarcinoma is yet to be verified. With the introduction of this new treatment strategy, the identification of patients with better response to neoadjuvant chemotherapy has become a crucial issue and many authors have proposed different methods for the evaluation of tumor regression after neoadjuvant chemotherapy, both in a radiologic or histopathologic setting (5,6). The purpose of this prospective multicenter cohort study is to verify the prognostic value of histopathological and radiological response to neoadjuvant chemotherapy in the treatment of locally advanced gastric adenocarcinoma with perioperative strategy. Oncological outcomes were also assessed.
Methods
Study population
From December 2009 through June 2015, all the patients with advanced gastric cancer, evaluated for perioperative chemotherapy at our institute were prospectively enrolled in this study. The inclusion criteria comprised histologically proven and locally advanced gastric adenocarcinoma (clinical ≥ T2 or nodal disease, M0), age 18–75 years and a WHO performance status of 0 or 1. Participants were required to have adequate renal (creatinine clearance >60 mL/min, serum creatinine <1.5 mg/dL), cardiac (ejection fraction >50%), liver (bilirubin level <1.5 mg/dL) and hematologic functions (WBC >4×109/L, ANC >2×109/L, platelets >100×109/L). Patients were excluded if there were radiological or clinical evidences of distance metastases or proven peritoneal carcinomatosis, if tumor clinical staging (cTNM) was less than II and if they had adenocarcinoma of distal esophagus. Finally, we excluded subjects who had previously received chemotherapy or radiotherapy. The study protocol was approved by the local ethics committee.
Staging and treatment
The initial work up included a physical general examination, standard laboratory tests, echocardiography, a digestive endoscopy with biopsy, an echo-endoscopy (EUS) and a computed tomography (CT) of thorax, mediastinum and abdomen. Clinical tumor staging (cTNM) was evaluated with the combination of CT and EUS and was determined according to the Seven Edition TNM classification (7). Patients received a chemotherapy regimen of ECF or ECX divided in 3 pre- and 3 post-operative cycles. Indeed, ECX regimen was found to be as effective as ECF and could reliably replace the latter (8). The treatment schedules were designed as follows: cisplatin (60 mg/m2 intravenously), epirubicin (50 mg/m2 intravenously) plus fluorouracil (200 mg/m2 daily for 21 days by continuous intravenous infusion) (ECF regimen) or capecitabine (500 mg/m2 orally two times a day) (ECX regimen). Surgery was performed three to 6 weeks after the third cycle of chemotherapy and a D2 subtotal/total gastrectomy was generally preferred. We performed D2 plus lymphadenectomy in patients at risk of lymph node metastases. Every surgical intervention were preceded by diagnostic laparoscopy to exclude the presence of peritoneal carcinomatosis or hepatic metastasis. Six to 12 weeks after surgical intervention patients underwent the last 3 cycles. Every cycle last 21 days, giving a total treatment length of 7 months. The severity of adverse effect associated with chemotherapy was defined according to the Common Terminology Criteria for Adverse Events (CTCAE v 4.0) (9).
Response evaluation and follow-up
In order to estimate the response to chemotherapy, patients underwent a second CT and volume change was assessed in accordance to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1) (10). The resected specimens were examined to determine the histological subtype (Lauren’s Classification), margin status (R0, R1 and R2) and definitive pathological TNM-derived stage. Additionally, the tissue sections were analyzed to define histological response to chemotherapy (Becker’s criteria). The tumor regression grading was based on percentage of vital tumor tissue compared to the identifiable tumor bed and was classified in three different grades: grade 1 (<10% residual tumor per tumor bed), grade 2 (10–50% residual tumor per tumor bed) and grade 3 (>50% residual tumor per tumor bed) (6). Follow-up assessment consists of physical examination, laboratory test (CA 19-9 and CEA), endoscopy, chest X-ray and abdominal and mediastinum CT scan. Participants were assessed every 3 months during the first year, then every 6 months during the second year and once yearly thereafter until the fifth year.
Statistical analysis
Patients’ data including epidemiologic, surgical, pathologic and survival figures, were prospectively compiled into a database (IBM SPSS® 20, 2012). Continuous data were expressed as median (range) and were compared with the Mann-Whitney U test; categorical data were expressed as percentage and were compared with the Fisher’s exact test. Survival rates were calculated using the Kaplan-Meier method and expressed as median (95% CI), and were compared using the log-rank test. P<0.05 was considered as statistically significant.
Results
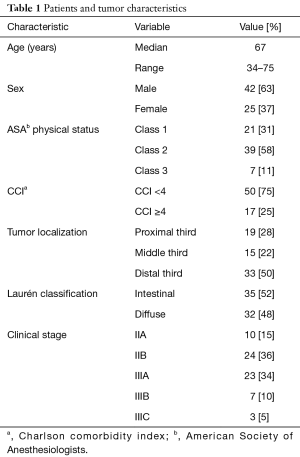
During the study period, a total of 156 patients with locally advanced adenocarcinoma were prospectively evaluated for this investigation; 49 patients were excluded due to their age (>75 years) or poor general condition, 13 subjects were excluded because of reduced ejection fraction and 10 patients were excluded in consequence of inadequate renal or liver function. Lastly, 17 patients were excluded because they already received chemotherapy or radiotherapy. Thus, 67 patients were finally included in the study. Patients and tumor characteristics are summarized in Table 1.
Full table
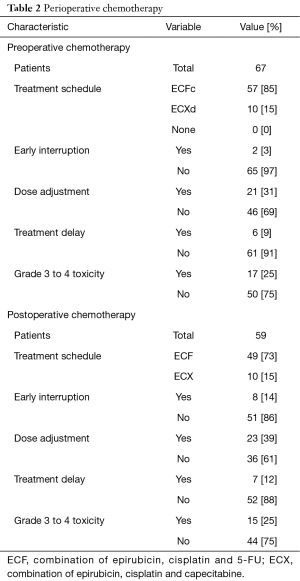
During first three pre-operative cycles, dose adjustments and treatment delays were required in 21 (31%) and 6 (9%) patients respectively, with two cases of early treatment interruption due to renal function decline. Drug-induced grade 3 to 4 toxicity rate was nearly 25% (17 subjects) (Table 2). After receiving the first three cycles of preoperative chemotherapy one patient did not proceeded to surgery because of peritoneal carcinomatosis discovered at the time of diagnostic laparoscopy. Therefore, only 66 people were considered suitable for surgical intervention.
Full table
Surgery was curative in 64 patients (R0), while two subjects presented microscopic deposits on resection margins (R1, infiltration in lesser omentum). There were 15 (23%) postoperative non-fatal complications, 13 of those treated conservatively and 2 managed by surgical intervention. The median hospital stay was 10 days (range, 6–49 days). None of the patients experienced intraoperative complications as well as readmission to hospital or death within 30 days. A total of 51 (86%) patients completed the following scheduled cycles successfully, 8 (14%) interrupted chemotherapy prematurely, whereas 8 subjects (12%) did not subsequently began adjuvant treatment because of poor conditions due to postoperative complications or prolonged hospital stay. A reduction from the starting dose of at least one of the chemotherapeutic agents was necessary for 23 (39%) people and 7 (12%) patients needed cycle delay. Nearly 25% [15] of the patients experienced severe chemotherapy-induced adverse effects (Table 2).
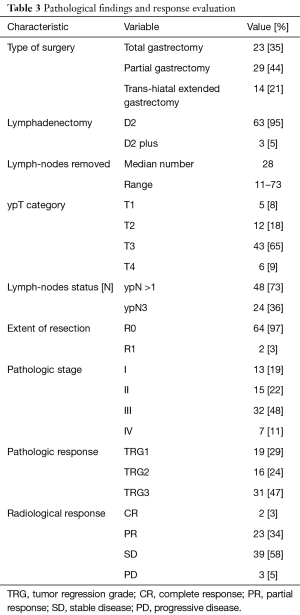
Surgical samples were analyzed to determine histological response to chemotherapy. The histopathological analysis showed 19 specimens with less than 10% of vital tumor left (grade 1), 16 specimens with grade 2 regression (10–50% residual tumor) and 31 specimens with more than 50% of vital tumor tissue compared to the identifiable tumor bed (grade 3). About volume change assessed by CT scan, 23 patients (34%) had a partial response, 39 patients (58%) had a disease stabilization and 3 (5%) subjects showed progressive disease. Only 2 patients (3%) had a CR. Pathological findings and response evaluation are listed in Table 3.
Full table
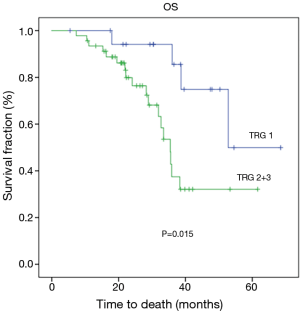
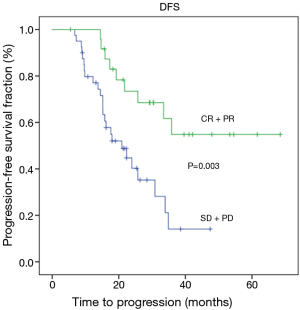
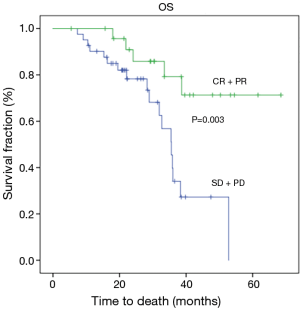
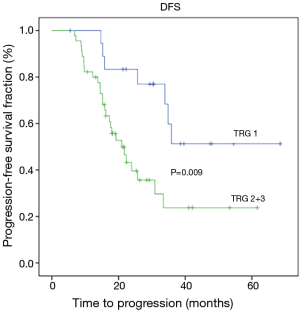
At the time of analysis, the median follow up was 27 months (range, 5.00–68.00 months). Twenty-three (34.3%) patients died due to disease progression, while 45 (65.7%) people were still alive and 31 (46.3%) had no evidence of disease. Of the 36 relapsed patients, 11 (30%) subjects developed peritoneal carcinomatosis and in 25 (70%) cases distant metastases or lymph node recurrence occurred. Median disease-free survival and over-all survival were 25.70 months (14.5–36.8) and 36.6 months (24.3–52.9), respectively. Radiological response was found to be significantly related to a better DFS (P=0.003) and OS (P=0.003) (Figures 1,2). Tumor regression grade (TRG) was significantly associated with DFS (P=0.009) and OS (P=0.015) (Figures 3,4). On the contrary histological type did not showed significant differences in terms of survival (P=0.229; P=0.383) (Table 4). Radiological response and histological regression grade were not statistically associated (P=0.333).
Full table
Discussion
Our results confirm that perioperative chemotherapy for locally advanced gastric adenocarcinoma is a feasible and safe treatment that can be reliably adopted in daily clinical practice; 66 of the 67 patients who started neoadjuvant treatment finally proceeded to surgery with a high curative resection rate (R0 97%) and an adequate treatment adherence (88% of them were able to receive both pre- and post-operative chemotherapy).
Perioperative chemotherapy has successfully been introduced in the treatment of locally advanced gastric adenocarcinoma. The MAGIC and FNCLCC/FFCD trials suggest that this strategy significantly increases patients survival when compared with surgery alone (5-year survival: MAGIC 36% vs. 23%, P=0.009; FNCLCC/FFCD 9703 38% vs. 24%, P=0.003) (3,4). Moreover, Ronellenfitsch et al. reported a higher R0 resection rate and better 5 years oncological outcomes in patients addressed to perioperative chemo (radio) therapy for resectable gastric adenocarcinoma (11).
On the other hand, a great criticism to the perioperative strategy is the delaying of surgery and the deterioration of patients during neoadjuvant chemotherapy, possibly resulting in poor surgical outcomes. However, the increase of radical resections in the perioperative treatment compared to surgery alone represents the main positive oncological prognostic factor up to now and therefore seems to actually justify the delay in surgery. From this point of view our study is in line with the MAGIC and FNCLCC/FFCD trials showing a considerable percentage of curative resections (97% of R0 rate). Surgical complications happened in 23% of the patients, similarly to those obtained by the French FNCLCC/FFCD study (25.7% of postoperative morbidity). In the MAGIC study, nearly all patients underwent gastrectomy with D1 lymphadenectomy, thus surgical findings and postoperative comorbidities are hardly comparable.
Our study depicts good results in terms of chemotherapy treatment adherence and related toxicity, showing the majority of patients completing the first three cycles of chemotherapy, while only 41.6% in MAGIC study and 50% in French study received planned postoperative cycles. The reason why such a small percentage of patients completed all protocol treatment was reasonably related to early disease progression, prolonged hospital stay or postoperative complications. On the contrary, nearly 75% of our subjects received all six cycles and 23 patients (34%) experienced grade 3–4 toxicity (41% 3–4 toxicity rate in FNCLCC/FFCD trial). These better results in terms of treatment adherence and chemotherapy-related toxicity are probably due to careful selection of candidates after precise inclusion criteria, which were described in our study protocol.
Even if baseline characteristics of our series slightly differs in terms of median age (67 years) and pathological tumor extension (48% of subjects with stage III tumors), the OS and DFS are comparable with those reported in the two trials (Table 4). These results confirm the survival benefit provided by perioperative strategy and show the crucial role of chemo-sensitivity in the treatment of gastric cancer. As previously mentioned, we concentrated our attention on radiological and histopathological tumor response after neoadjuvant chemotherapy. We found better results in terms of both DSF and OS in patients with a good radiological response to chemotherapy assessed by CT, as we reported above (Figures 1,2) (Table 4). The prognostic value of radiological response has been already described in previous studies (5,12,13), however its significance in patients with gastric cancer must be valued with considerable caution. Indeed, chemotherapy-induced inflammation and edema can distort the layers of the stomach wall and can lead to errors in evaluating the depth of tumor invasion (14). Another important aspect to consider is the inability of CT to differentiate chemotherapy induced fibrosis from vital tumor, often leading to an underestimation of the residual tumor size (14,15).
Additionally, in our series, radiological response assessed by CT images shows no correlation with pathological regression (P=0.333), suggesting the considerable limitations of this post-treatment evaluation method.
Regarding histopathological response (Becker’s criteria), our series shows 29% of patients with total or sub-total response (TRG1), and these data are consistent with those obtained in the two main retrospective studies concerning TRG (21% and 24% of TRG1 in Becker’s and in Schmidt’s study, respectively) (12,16). Differently, in two recent retrospective works regarding predictor factors of survival after perioperative MAGIC-style chemotherapy, TRG1 rates were lower (17%) (17,18). In Becker’s analysis TRG was significantly correlated with survival, while in Schmidt’s study TRG does not reveal itself as an independent prognostic factor. In our investigation, TRG shows significant correlation with both DFS and OS (Figures 3,4) (Table 4), suggesting that patients with complete or subtotal regression have a better outcome. Even if its independent value as a prognostic marker still remain a matter of debate, TRG can be considered an unique in vivo assessment of chemosensitivity and might be used as a crucial criterion for tailored post-operative treatment. Another aspect to consider is that the biological behavior of gastric adenocarcinoma differs significantly upon its primary anatomic localization and histological subtyping according to Lauren’s classification (19). Nowadays, the effectiveness of perioperative chemotherapy for the treatment of signet ring cell adenocarcinoma is a matter of debate (20). In a recent multicenter comparative cohort study, Messanger et al. affirms that perioperative chemotherapy provides no survival benefit in patients with diffuse type carcinoma and suggests that a policy of primary surgery followed by adjuvant chemotherapy should be considered as standard therapy for this type of tumor (21). Our experience indicates no difference in terms of DFS and OS between diffuse and intestinal carcinoma and both Lauren types present comparable results in terms of TRG and clinical response (Table 4). An ongoing phase II/III controlled randomized trial (22), in which perioperative treatment is compared to a strategy of primary surgery followed by adjuvant chemotherapy for locally advanced signet ring cell carcinoma, will probably provide more evidences for a different approach for this type of tumor in the next years. Today perioperative chemotherapy remains the standard of care.
Despite the small number of participants treated, our prospective analysis shows that selected patients with local advanced adenocarcinoma can be safely managed with perioperative chemotherapy in daily clinical practice and our results confirm the survival benefit and the feasibility of perioperative treatment highlighted by the two main trials found in literature. Both TRG and radiological response can be considered promising marker of chemotherapy response and we underline the need for randomized trials dedicated to this histopathologic and radiologic analysis in order to establish their independent value and clinical application. Considering the proved survival benefit provided by neoadjuvant and adjuvant therapies for gastric adenocarcinoma, future studies should investigate predictive markers of response to chemotherapy in order to identify which patients are most likely to benefit from this treatment modality.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the scientific ethics committee of Niguarda Ca’ Granda Hospital and written informed consent was obtained from all the patients.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [Crossref] [PubMed]
- Siewert JR, Böttcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998;228:449-61. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Ott K, Sendler A, Becker K, et al. Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric Cancer 2003;6:159-67. [Crossref] [PubMed]
- Becker K, Mueller JD, Schulmacher C, et al. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 2003;98:1521-30. [Crossref] [PubMed]
- Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7 ed. New York: Springer, 2010.
- Ocvirk J, Reberšek M, Skof E, et al. Randomized prospective phase II study to compare the combination chemotherapy regimen epirubicin, cisplatin, and 5-fluorouracil with epirubicin, cisplatin, and capecitabine in patients with advanced or metastatic gastric cancer. Am J Clin Oncol 2012;35:237-41. [Crossref] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) v 4.0.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Ronellenfitsch U, Schwarzbach M, Hofheinz R, et al. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst Rev 2013.CD008107. [PubMed]
- Schmidt T, Sicic L, Blank S, et al. Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer 2014;110:1712-20. [Crossref] [PubMed]
- Molina R, Lamarca A, Martínez-Amores B, et al. Perioperative chemotherapy for resectable gastroesophageal cancer: a single-center experience. Eur J Surg Oncol 2013;39:814-22. [Crossref] [PubMed]
- Park SR, Lee JS, Kim CG, et al. Endoscopic ultrasound and computed tomography in restaging and predicting prognosis after neoadjuvant chemotherapy in patients with locally advanced gastric cancer. Cancer 2008;112:2368-76. [Crossref] [PubMed]
- Yoshikawa T, Tanabe K, Nishikawa K, et al. Accuracy of CT staging of locally advanced gastric cancer after neoadjuvant chemotherapy: cohort evaluation within a randomized phase II study. Ann Surg Oncol 2014;21 Suppl 3:S385-9. [Crossref] [PubMed]
- Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 2011;253:934-9. [Crossref] [PubMed]
- Blackham AU, Greenleaf E, Yamamoto M, et al. Tumor regression grade in gastric cancer: Predictors and impact on outcome. J Surg Oncol 2016;114:434-9. [Crossref] [PubMed]
- Mingol F, Gallego J, Orduña A, et al. Tumor regression and survival after perioperative MAGIC-style chemotherapy in carcinoma of the stomach and gastroesophageal junction. BMC Surg 2015;15:66. [Crossref] [PubMed]
- Reim D, Gertler R, Novotny A, et al. Adenocarcinomas of the esophagogastric junction are more likely to respond to preoperative chemotherapy than distal gastric cancer. Ann Surg Oncol 2012;19:2108-18. [Crossref] [PubMed]
- Blank S, Stange A, Sisic L, et al. Preoperative therapy of esophagogastric cancer: the problem of nonresponding patients. Langenbecks Arch Surg 2013;398:211-20. [Crossref] [PubMed]
- Messager M, Lefevre JH, Pichot-Delahaye V, et al. The impact of perioperative chemotherapy on survival in patients with gastric signet ring cell adenocarcinoma: a multicenter comparative study. Ann Surg 2011;254:684-93. [Crossref] [PubMed]
- Piessen G, Messager M, Le Malicot K, et al. Phase II/III multicentre randomised controlled trial evaluating a strategy of primary surgery and adjuvant chemotherapy versus peri-operative chemotherapy for resectable gastric signet ring cell adenocarcinomas - PRODIGE 19 - FFCD1103 - ADCI002. BMC Cancer 2013;13:281. [Crossref] [PubMed]