Case report: primary acinar cell carcinoma of the liver treated with multimodality therapy
Acinar cell carcinoma (ACC) of the pancreas is uncommon and represents approximately 1–2% of all pancreas cancers (1-3). It is a pancreatic exocrine malignancy that is characterized by the expression of enzymes such as trypsin, chymotrypsin, amylase and lipase, detectable by immunohistochemistry (1). ACC of the pancreas differs from pancreatic ductal adenocarcinoma (PDAC) not only clinically and pathologically but also in terms of molecular features (4). In PDAC alterations in genes such as TP53 (tumor protein 53), KRAS (V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog), DPC4 (deleted in pancreatic cancer 4), p16 are commonly observed whereas in ACC they are rare. In ACC aberrations in the APC/β-catenin pathway and losses on chromosome 11 have been reported (5).
ACC of the pancreas is generally associated with a relatively improved prognosis as compared to PDAC, but is more aggressive when compared to endocrine pancreatic tumors (6). It is more commonly seen in males in the fifth or sixth decade (1,4-6). A paraneoplastic phenomenon with an elevated lipase known as lipase hypersecretion syndrome can also occur and may be associated with cutaneous findings (7,8).
Extra pancreatic ACCs are infrequent but have been described previously in the jejunum, stomach and periampullary region of duodenum, and which are most commonly assumed to occur due to the presence of pancreatic heterotopia (9-15). Primary ACC of the liver is extremely rare and has been reported very rarely in the literature. Prior publications include a case report of a 35-year-old lady with an elevated serum alpha fetoprotein (AFP) treated with a left hepatic lobectomy, while a second report included four primary liver tumors of ACC type in which all four were treated surgically (16). One of the patients developed distant recurrence and was treated with chemotherapy (17). A third report commented on imaging findings identified in primary ACC of the liver (18). We report herein a case of primary ACC of the liver in a young woman, with bilobar liver disease at presentation treated with combination of surgical resection, hepatic arterial embolization and chemotherapy who had molecular profiling of her tumor.
Case presentation
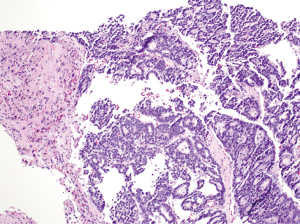
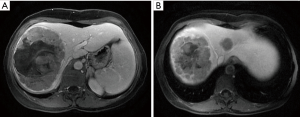
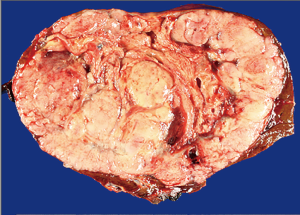
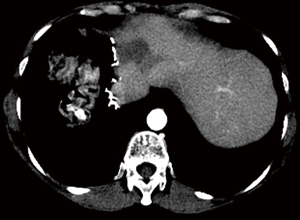
A 54-year-old female former one pack year smoker with no comorbidities was treated with terbinafine (Lamisil) for a fungal foot infection and was noted to early satiety and abdominal discomfort and blood testing demonstrated elevated transaminases. Transaminitis persisted following discontinuation of terbinafine, and an abdominal ultrasound was performed which showed a right liver mass. MRI liver with gadoxetate disodium contrast was performed to further evaluate, and demonstrated two liver masses; a hypoenhancing mass occupying the right hepatic lobe measuring 12.9 cm × 10.4 cm × 14.2 cm causing narrowing of the right hepatic vein and a smaller mass in segment IVA measuring 3.0 cm × 2.8 cm (see Figure 1A,B). Laboratory investigations revealed a CEA of 1.3 ng/mL (0–5 ng/mL), CA19-9 38 U/mL (0–40 U/mL) and elevated AFP 98.7 ng/mL (0–5 ng/mL). Further imaging with CT revealed no extra-hepatic sites of disease and specifically the pancreas appeared normal. Hepatitis serology was negative and a recent colonoscopy was normal. A right partial hepatectomy was performed to remove the dominant mass, however the second mass in segment IVA was found to be unresectable due to its proximity to the left hepatic vein. A biopsy of the second lesion was taken at the time of surgery. Pathological review of the resected tumor specimen revealed a carcinoma with a maximal tumor dimension of 16.3 cm (Figure 2). Microscopically, the carcinoma was characterized by acinar structures consists of cells of various sizes with eosinophilic cytoplasm and basally located nuclei (Figure 3A,B). Lymphovascular invasion was identified. The adjacent hepatic parenchyma had evidence of non-specific reactive changes with no significant fibrosis or cirrhosis. The biopsy of the second unresectable tumor was morphologically similar to the resected dominant lesion (Figure 4). Immunohistochemical staining showed positivity for trypsin (Figure 5A), chymotrypsin and focal staining for CK19. The tumor cells were negative for Hep Par-1 (Figure 5B), ER, PR and TTF-1. Chromogranin and synaptophysin staining showed only scattered individual cell positivity in the tumor resection specimen.
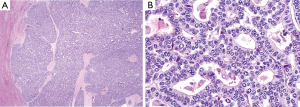
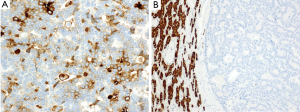
Given the morphological and immuno typical features and no evidence of another primary source the diagnosis was consistent with an ACC involving the liver. A postoperative CT scan showed an increase in the segment IVA mass now measuring 7.2 cm × 4.8 cm with no new liver lesions or distant sites of disease evident. The patient proceeded to hepatic arterial embolization of the segment IVA lesion, which was performed using 100–300 µm Embosphere microspheres. A decision was then made for FOLFOX chemotherapy for six cycles following embolization with a view to treatment of residual micrometastatic disease. The patient completed FOLFOX therapy with no significant toxicity and recent imaging demonstrates a decrease in size of the necrotic tumor in segment IVA (3.8 cm × 2.8 cm) with no other evidence of disease (Figure 6). At time of manuscript submission, twenty months since diagnosis, the patient remains well and is currently under observation. Genomic profiling was also performed on the resected specimen using an in-house exon capture assay (Integrated Mutation Profiling of Actionable Cancer Targets, or MSK-IMPACT) to interrogate the mutation and copy number status of 379 oncogenes and tumor suppressor genes of the tumor compared to matched normal blood. Results revealed a β-catenin S45P mutation, deletions in chromosome 11, a MAP2K4 deletion and a CDH1 truncation mutation (see Table 1).
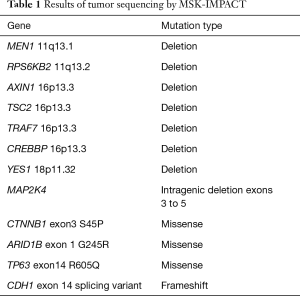
Full table
Discussion
ACC is a rare neoplasm with distinct characteristics that help aid its diagnosis. There were several radiographic and histologic features in this case that helped determine the diagnosis of primary ACC of the liver. On imaging both masses were hypo-enhancing with the larger lesion having evidence of necrosis. These features have been previously documented in ACC, with the presence of necrosis usually seen in larger lesions while smaller lesions are usually more solid in appearance (18,19). Primary ACCs most frequently metastasizes to the liver, therefore it is important to clearly establish that the liver lesions are not representative of metastatic disease from a pancreas ACC (1). In our patient there was no evidence of a pancreatic mass on imaging, at time of surgery, and the pancreas remains normal in appearance on current surveillance imaging 18 months since diagnosis. Morphologically the resected tumor and biopsy from the second lesion were consistent with ACC. Immunohistochemistry was of utmost importance in aiding in the diagnosis. The important diagnostic feature was the positivity by IHC for trypsin and chymotrypsin which have a 95% sensitivity to detect acinar differentiation (5). The tumor did have focal staining for CK19 and CK7 which are ductal epithelial markers. Presence of CK19 and CK7 has been described in ACCs with CK19 staining occurring in 86% and CK7 staining in 73% in one series (20). This finding along with other reports corroborates the presence of CK7 and CK19 staining in ACCs (21,22). Given our patient’s tumor lacked the hepatocellular marker Hep-Par-1, the adjacent liver in the surgical specimen was normal with no evidence of cirrhosis, had no clinical risk factors for hepatitis and hepatitis serology was negative, these factors all point against a diagnosis of hepatocellular carcinoma. Our patient did have a slightly elevated AFP prior to surgery; this phenomenon has also been described in primary ACC of the pancreas and of the liver previously (16,22). Therefore in summary with regards to other differential diagnoses such as cholangiocarcinoma or multifocal hepatoma our case has features which are clearly consistent with ACC.
Elevated lipase can occur in ACC leading to a paraneoplastic syndrome with patients having evidence of panniculitis, eosinophilia and subcutaneous fat necrosis (8,23). This phenomenon also known as lipase hypersecretion syndrome can have an associated elevated amylase and patients tend to have a more aggressive clinical trajectory (8). In our patient lipase was normal; 41 U/L (range, 21–55 U/L) at diagnosis and clinically has had no evidence of such a syndrome throughout her disease course to date.
Surgery is the definitive treatment for localized ACC. Because one of the lesions was unresectable due to its proximity to the left hepatic vein, hepatic arterial embolization was performed following resection of the larger lesion. An excellent response to this procedure was evident, with central necrosis and a reduction in size of the lesion seen on imaging five weeks post embolization procedure and its effect continues on most recent imaging. There is a paucity of data regarding the efficacy of systemic therapy in ACC with gemcitabine or 5 fluorouracil based combination therapy most frequently utilized (4). In our case FOLFOX was administered for six cycles following embolization. Prognostic factors in ACC are not well established; stage has been shown to be prognostic with vascular invasion, perineural invasion, CK19 having a trend to a poorer prognosis (20,24).
This patient had no prior personal history of malignancy, and her family history was unknown as she was adopted. The association of other tumor types with ACC is unclear; although in one report 25% cases (10 patients) had a prior personal history of malignancy (4). The relationship of ACC development to inherited cancer predisposition syndromes is also uncertain, although cases have been reported to occur in a BRCA1 germline carrier and Lynch syndrome (4,25,26). Mismatch repair (MMR) deficiency has been seen to occur in ACC of the pancreas (26,27). The presence or absence of MMR staining was not commented on in the previous reported cases of primary ACC of the liver. In our case immunohistochemical staining revealed that DNA MMR protein is retained in the tumor.
Ectopic pancreas tissue has been suggested as contributing to the development of extra pancreatic ACC (10,14). Ectopic pancreas tissue has been illustrated in a case report as occurring in conjunction with a choledochal cyst and abnormal biliary anatomy (28). In our case there was no suggestion of such any such anatomical abnormalities present. In the primary ACC of the liver cases previously described in the literature none of the cases had heterotopic pancreas tissue evident. Pancreatic and liver cells are known to arise from a mutual progenitor cell and research has shown the capability of liver cells to differentiate to pancreatic type cells (29-31). Generating a hypothesis in this case that could have caused our patients tumor to develop it is possible that it is due to aberrant development of a common progenitor cell or given the presence of focal staining of cytokeratins CK7 and CK19 in our patient’s tumor possible transformation of a ductal epithelial cell with pure acinar differentiation.
Given the rarity of ACC it has been here-to-fore difficult to characterize the molecular profile of ACC however given the increased ability to perform tumor genomic sequencing this information is becoming more accessible. Genomic alterations found in pancreatic adenocarcinoma are different compared to ACC with mutations in KRAS, TP53, DPC4 and p16 commonly seen in PAC but are rarely found in ACC (4). Alterations in the adenomatosis polyposis coli-beta-catenin (APC-β catenin) pathway and allelic loss on chromosome 11p were previously found in ACC of the pancreas (27,32).
In our sequencing results deletions were evident in MEN1 and RPS6KB2 which are located on the long arm (q) of chromosome 11. Deletions were also seen on the short arm (p) of chromosome 16 in our case with AXIN1, TSC2, TRAF7 and CREBBP known to be located at this site. Loss of heterozygosity at chromosomes 11q, 13q, 15q and 16q in nine pancreatic ACCs was previously reported (33). A frameshift alteration resulting in a CDH1 (E cadherin) truncating mutation on chromosome 16q was also present in our case. Associated with lobular breast cancer and gastric carcinoma CDH1 somatic alterations have not been documented in ACC. In a previous report all 7 cases of ACC of the pancreas had E cadherin staining (34). A MAP2K4 (Mitogen activated protein kinase kinase 4) intragenic deletion was detected in our sequencing results for this tumor. MAP2K4 is located on the short arm of chromosome 17 and alterations in this gene have been described in high grade serous and endometrioid cancers of the ovary, lung and pancreas carcinoma (35-37). Our case we believe is the first to describe this type of alteration in ACC.
Other mutations such germline or somatic mutations in BRCA2 and FAT genes have been described to be present in ACC with one patient with liver metastases who had a BRCA2 mutation obtaining a complete response to cisplatin based chemotherapy (38). DCC (deleted in colon cancer) and c-Myc alterations have been observed, with 30.4% (17/56) tumors showing complete loss of DCC expression and 48.2% (27/56) tumors having significant reduction of DCC expression while 17% had c-Myc amplification in one series (39). Our patient did not have evidence of DCC, c-Myc amplification or a BRCA2 somatic mutation (germline testing not performed) although we must make note these previous studies were of pancreatic ACCs. SND1-BRAF fusions have been reported to occur in 23% of a 44 sample set of ACCs of the pancreas which could allow possible MEK inhibition as a therapeutic strategy (40). Neither this alteration nor any other type of BRAF fusion was detected in our patient by MSK-IMPACT.
Our case is the first to describe the somatic molecular alterations seen in a primary ACC of the liver. Our patient had a mutation in CTNNB1 (β-catenin) S45P which results in an amino acid substitution at position 45 of β-catenin from a serine to a proline which is notably involved in β-catenin phosphorylation and ubiquitination (41). This mutation occurs on the glycogen synthase kinase 3 beta (GSK3β) phosphorylation site within β-catenin which allows β-catenin stabilization, leading to constitutive transcription activation of β-catenin/T-cell factor/lymphoid enhancer factor (TCF/LEF) genes in the Wnt signaling pathway (42,43). This mutation has been described in adrenocortical adenomas, adrenocortical carcinomas and hepatocellular carcinoma (34,42,44-46). The CTNNB1 S45P mutation reflects the likely importance of Wnt signaling in this case.
In summary, we describe a rare case of primary ACC of the liver, highlighting the importance of multidisciplinary approach to management. Molecular profiling of this identified several possible molecular drivers, including Wnt signaling, deletions in chromosome 11 and 16. We also saw a CDH1 truncating mutation and a MAP2K4 deletion. Targeting the Wnt signaling pathway may have role in future therapeutic strategies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Klimstra DS, Heffess CS, Oertel JE, et al. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am J Surg Pathol 1992;16:815-37. [Crossref] [PubMed]
- Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol 2006;12:3180-5. [Crossref] [PubMed]
- Wisnoski NC, Townsend CM Jr, Nealon WH, et al. 672 patients with acinar cell carcinoma of the pancreas: a population-based comparison to pancreatic adenocarcinoma. Surgery 2008;144:141-8. [Crossref] [PubMed]
- Lowery MA, Klimstra DS, Shia J, et al. Acinar cell carcinoma of the pancreas: new genetic and treatment insights into a rare malignancy. Oncologist 2011;16:1714-20. [Crossref] [PubMed]
- Klimstra DS. Nonductal neoplasms of the pancreas. Mod Pathol 2007;20 Suppl 1:S94-112. [Crossref] [PubMed]
- Holen KD, Klimstra DS, Hummer A, et al. Clinical characteristics and outcomes from an institutional series of acinar cell carcinoma of the pancreas and related tumors. J Clin Oncol 2002;20:4673-8. [Crossref] [PubMed]
- Riediger C, Mayr M, Berger H, et al. Transarterial chemoembolization of liver metastases as symptomatic therapy of lipase hypersecretion syndrome. J Clin Oncol 2012;30:e209-12. [Crossref] [PubMed]
- Moro M, Moletta L, Blandamura S, et al. Acinar cell carcinoma of the pancreas associated with subcutaneous panniculitis. JOP 2011;12:292-6. [PubMed]
- Makhlouf HR, Almeida JL, Sobin LH. Carcinoma in jejunal pancreatic heterotopia. Arch Pathol Lab Med 1999;123:707-11. [PubMed]
- Mizuno Y, Sumi Y, Nachi S, et al. Acinar cell carcinoma arising from an ectopic pancreas. Surg Today 2007;37:704-7. [Crossref] [PubMed]
- Sun Y, Wasserman PG. Acinar cell carcinoma arising in the stomach: a case report with literature review. Hum Pathol 2004;35:263-5. [Crossref] [PubMed]
- Fukunaga M. Gastric carcinoma resembling pancreatic mixed acinar-endocrine carcinoma. Hum Pathol 2002;33:569-73. [Crossref] [PubMed]
- Coyne JD. Pure pancreatic-type acinar cell carcinoma of the stomach: a case report. Int J Surg Pathol 2012;20:71-3. [Crossref] [PubMed]
- Ambrosini-Spaltro A, Poti O, De Palma M, et al. Pancreatic-type acinar cell carcinoma of the stomach beneath a focus of pancreatic metaplasia of the gastric mucosa. Hum Pathol 2009;40:746-9. [Crossref] [PubMed]
- Moncur JT, Lacy BE, Longnecker DS. Mixed acinar-endocrine carcinoma arising in the ampulla of Vater. Hum Pathol 2002;33:449-51. [Crossref] [PubMed]
- Hervieu V, Lombard-Bohas C, Dumortier J, et al. Primary acinar cell carcinoma of the liver. Virchows Arch 2008;452:337-41. [Crossref] [PubMed]
- Agaimy A, Kaiser A, Becker K, et al. Pancreatic-type acinar cell carcinoma of the liver: a clinicopathologic study of four patients. Mod Pathol 2011;24:1620-6. [Crossref] [PubMed]
- Tatli S, Mortele KJ, Levy AD, et al. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am J Roentgenol 2005;184:511-9. [Crossref] [PubMed]
- Hammond NA, Miller FH, Day K, et al. Imaging features of the less common pancreatic masses. Abdom Imaging 2013;38:561-72. [Crossref] [PubMed]
- La Rosa S, Adsay V, Albarello L, et al. Clinicopathologic study of 62 acinar cell carcinomas of the pancreas: insights into the morphology and immunophenotype and search for prognostic markers. Am J Surg Pathol 2012;36:1782-95. [Crossref] [PubMed]
- Hoorens A, Prenzel K, Lemoine NR, et al. Undifferentiated carcinoma of the pancreas: analysis of intermediate filament profile and Ki-ras mutations provides evidence of a ductal origin. J Pathol 1998;185:53-60. [Crossref] [PubMed]
- Notohara K, Hamazaki S, Tsukayama C, et al. Solid-pseudopapillary tumor of the pancreas: immunohistochemical localization of neuroendocrine markers and CD10. Am J Surg Pathol 2000;24:1361-71. [Crossref] [PubMed]
- Potts DE, Mass MF, Iseman MD. Syndrome and pancreatic disease, subcutaneous fat necrosis and polyserositis. Case report and review of literature. Am J Med 1975;58:417-23. [Crossref] [PubMed]
- La Rosa S, Sessa F, Capella C. Acinar Cell Carcinoma of the Pancreas: Overview of Clinicopathologic Features and Insights into the Molecular Pathology. Front Med (Lausanne) 2015;2:41. [Crossref] [PubMed]
- Karamurzin Y, Zeng Z, Stadler ZK, et al. Unusual DNA mismatch repair-deficient tumors in Lynch syndrome: a report of new cases and review of the literature. Hum Pathol 2012;43:1677-87. [Crossref] [PubMed]
- Liu W, Shia J, Gonen M, et al. DNA mismatch repair abnormalities in acinar cell carcinoma of the pancreas: frequency and clinical significance. Pancreas 2014;43:1264-70. [Crossref] [PubMed]
- Abraham SC, Wu TT, Hruban RH, et al. Genetic and immunohistochemical analysis of pancreatic acinar cell carcinoma: frequent allelic loss on chromosome 11p and alterations in the APC/beta-catenin pathway. Am J Pathol 2002;160:953-62. [Crossref] [PubMed]
- Bahadir B, Ozdamar SO, Gun BD, et al. Ectopic pancreas associated with choledochal cyst and multiseptate gallbladder. Pediatr Dev Pathol 2006;9:312-5. [Crossref] [PubMed]
- Deutsch G, Jung J, Zheng M, et al. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development 2001;128:871-81. [PubMed]
- Ber I, Shternhall K, Perl S, et al. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem 2003;278:31950-7. [Crossref] [PubMed]
- Meivar-Levy I, Ferber S. New organs from our own tissues: liver-to-pancreas transdifferentiation. Trends Endocrinol Metab 2003;14:460-6. [Crossref] [PubMed]
- Furlan D, Sahnane N, Bernasconi B, et al. APC alterations are frequently involved in the pathogenesis of acinar cell carcinoma of the pancreas, mainly through gene loss and promoter hypermethylation. Virchows Arch 2014;464:553-64. [Crossref] [PubMed]
- Rigaud G, Moore PS, Zamboni G, et al. Allelotype of pancreatic acinar cell carcinoma. Int J Cancer 2000;88:772-7. [Crossref] [PubMed]
- Comper F, Antonello D, Beghelli S, et al. Expression pattern of claudins 5 and 7 distinguishes solid-pseudopapillary from pancreatoblastoma, acinar cell and endocrine tumors of the pancreas. Am J Surg Pathol 2009;33:768-74. [Crossref] [PubMed]
- Davis SJ, Choong DY, Ramakrishna M, et al. Analysis of the mitogen-activated protein kinase kinase 4 (MAP2K4) tumor suppressor gene in ovarian cancer. BMC Cancer 2011;11:173. [Crossref] [PubMed]
- Ahn YH, Yang Y, Gibbons DL, et al. Map2k4 functions as a tumor suppressor in lung adenocarcinoma and inhibits tumor cell invasion by decreasing peroxisome proliferator-activated receptor gamma2 expression. Mol Cell Biol 2011;31:4270-85. [Crossref] [PubMed]
- Xin W, Yun KJ, Ricci F, et al. MAP2K4/MKK4 expression in pancreatic cancer: genetic validation of immunohistochemistry and relationship to disease course. Clin Cancer Res 2004;10:8516-20. [Crossref] [PubMed]
- Furukawa T, Sakamoto H, Takeuchi S, et al. Whole exome sequencing reveals recurrent mutations in BRCA2 and FAT genes in acinar cell carcinomas of the pancreas. Sci Rep 2015;5:8829. [Crossref] [PubMed]
- Bergmann F, Aulmann S, Sipos B, et al. Acinar cell carcinomas of the pancreas: a molecular analysis in a series of 57 cases. Virchows Arch 2014;465:661-72. [Crossref] [PubMed]
- Chmielecki J, Hutchinson KE, Frampton GM, et al. Comprehensive genomic profiling of pancreatic acinar cell carcinomas identifies recurrent RAF fusions and frequent inactivation of DNA repair genes. Cancer Discov 2014;4:1398-405. [Crossref] [PubMed]
- Suarez MI, Uribe D, Jaramillo CM, et al. Wnt/beta-catenin signaling pathway in hepatocellular carcinomas cases from Colombia. Ann Hepatol 2015;14:64-74. [PubMed]
- Tissier F, Cavard C, Groussin L, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res 2005;65:7622-7. [Crossref] [PubMed]
- Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol 2012;4. [Crossref] [PubMed]
- Gaujoux S, Hantel C, Launay P, et al. Silencing mutated beta-catenin inhibits cell proliferation and stimulates apoptosis in the adrenocortical cancer cell line H295R. PLoS One 2013;8:e55743. [Crossref] [PubMed]
- Pinto EM, Chen X, Easton J, et al. Genomic landscape of paediatric adrenocortical tumours. 2015;6:6302.
- Mullen JT, DeLaney TF, Rosenberg AE, et al. beta-Catenin mutation status and outcomes in sporadic desmoid tumors. Oncologist 2013;18:1043-9. [Crossref] [PubMed]