Predictors of intermediate-term survival with destination locoregional therapy of hepatocellular cancer in patients either ineligible or unwilling for liver transplantation
Introduction
The incidence of hepatocellular carcinoma (HCC) has increased dramatically, with deaths secondary to liver cancer rising at a rate higher than that of any other malignancy (1). Importantly, HCC prevalence is predicted to increase among the geriatric population as efforts directed at birth cohort screening for chronic hepatitis C virus (HCV) and recognition of the global epidemic of Nonalcoholic Fatty Liver Disease (NAFLD) have led to increased screening (2). Curative therapies for HCC include segmental hepatic resection and liver transplant (LT), with 5-year overall survival rates ranging from 27 to 70 percent and 44 to 78 percent, respectively (3).
Elderly patients (age greater than 60) are often not deemed candidates for resection or LT due to coexisting cardiopulmonary disease, other organ dysfunction, or psychosocial comorbidity that renders candidacy for operative therapy marginal. Locoregional therapies (LRT), including intra-arterial: transarterial chemoembolization (TACE) or transarterial radioembolization (Y-90); as well as percutaneous: radiofrequency ablation (RFA), microwave ablation (MWA), and external beam radiation therapy (EBRT), are often employed as “destination therapy” for patients who are not candidates for or are unwilling to undergo surgical resection or LT. Use in this context is in contrast to “bridging,” in which such therapy is employed to maintain or downstage potential LT candidates within T2/Milan criteria.
While data regarding predictors of response and survival en route to transplant have been described (4,5), less is known regarding utility and predictors of response to locoregional therapy when employed as destination therapy in elderly populations. The aim of this investigation was to evaluate the use of locoregional approaches as destination therapy for HCC in an elderly population within criteria but either ineligible or unwilling for transplant and to investigate predictors of short (1-year) and intermediate (3-year) term survival.
Methods
Study design
This was a retrospective cohort study in which 123 patients over the age of 60 receiving destination locoregional therapy for HCC were reviewed and evaluated for predictors of 1- and 3-year survival. After institutional board approval, chart review was conducted on patients presenting to a university-based transplant clinic for diagnosis of HCC between January 2009 and January 2013.
Inclusion criteria
Inclusion criteria included: age greater than 60, HCC diagnosis made by multiphasic cross-sectional imaging (triphasic CT or MRI with Eovist), initial tumor burden within T2/Milan criteria, and use of locoregional interventions as destination therapy rather than bridging. When applicable, patients were offered transplant evaluation, though all were ultimately denied for various reasons by a multidisciplinary transplant committee.
Demographics, tumor and treatment variables
Patient demographics, liver disease factors, and tumor treatment characteristics were compared between the cohort surviving 3 years from initiation of locoregional therapy and the cohort that did not. Patient demographics included gender, age, ethnicity, whether LT evaluation was pursued, and reason for LT denial. Liver disease factors reviewed included etiology of cirrhosis and albumin, total bilirubin, INR, and creatinine at diagnosis, as well as model for end-stage liver disease (MELD) at diagnosis and after locoregional therapy. Tumor and treatment characteristics included number of lesions, AFP at diagnosis, presence of unilobar disease, and initial treatment modality.
Treatment modality was based on imaging review and discretion of the interventional radiologists. Modalities employed included TACE, Y-90, RFA, MWA, and EBRT as described elsewhere (6,7).
Statistical analysis
Continuous variables were examined with Levene’s test for equal variances and student’s t-test. Categorical variables were examined using Fisher’s exact test and Pearson’s chi square analysis; P<0.05 was considered as significant. Statistical Product and Services Solutions (SPSS, version 21, Chicago, IL, USA) was used for statistical analyses.
Results
Intermediate survival with destination HCC therapy and reasons for transplant ineligibility
One hundred and twenty-three patients with HCC met inclusion criteria, with 50/123 (40.6%) alive at 3 years. No significant difference existed between the cohort surviving 3 years and the remaining population with respect to age at presentation (P=0.232), gender (P=0.065), or ethnicity (P=0.503) (Table 1). Forty-eight percent of the cohort surviving 3 years and 22% of the population that did not (P=0.030) underwent evaluation for transplant and were ultimately denied listing for similar reasons, including (cohort alive at 3 years vs. not): severe cardiopulmonary disease (38.2% vs. 36.1%), active substance abuse (17.6% vs. 21.3%), psychiatric (8.8% vs. 12.7%), unwilling (26.5% vs. 12.9%) and other (8.8% vs. 17%) (P=0.764) (Table 1).
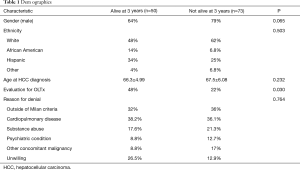
Full table
Tumor characteristics/treatment modalities
No significant difference was found between the two groups in terms of tumor characteristics including number of lesions at diagnosis (P=0.156), AFP at diagnosis (P=0.898), and presence of unilobar disease (P=0.540). Initial treatment modality employed, including TACE, Y-90, RFA, MWA, bland transarterial embolization, and EBRT, was also not found to be significant (P=0.760) (Table 2).
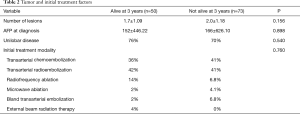
Full table
Liver disease factors and intermediate survival
No significant differences existed between groups with respect to liver disease factors including etiology of liver disease (P=0.113), albumin (P=0.376), bilirubin (P=0.354), or creatinine at diagnosis (P=0.708). However, INR at diagnosis was significant (P=0.024) (Table 3). In addition, the cohort surviving 3 years had a significantly lower MELD score at HCC diagnosis (9.7 vs. 11.4, P=0.037) and MELD following initial locoregional therapy (10.7 vs. 13.3, P=0.008) (Table 3).
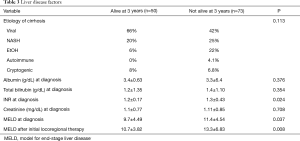
Full table
Discussion
Inherent to a global trend towards an increasing average lifespan comes an increasingly elderly population diagnosed with HCC (8). This has given rise to ongoing debate as to the most appropriate treatment strategies for this population. Numerous treatment options are available, with transplant allocation determined based on a comprehensive assessment of candidacy and individual fitness for surgery. Available treatment options include potentially curative therapies such as LT or surgical resection, ablative techniques, chemoembolization, radioembolization (Y-90), stereotactic radiation, and chemotherapy. There is significant interest in developing effective and durable treatment regimens for those who do not undergo LT or surgical resection. Elderly patients often are not candidates for surgery and there is increasing prevalence of HCC and liver-related morbidity in this population.
Advances in LRT and techniques have allowed for effective treatments with notable anti-tumor or oncostatic effect, safety, and minimal invasiveness (when compared to surgery), and represent an alternative therapeutic option (9). Per guidelines by the American Association for the Study of Liver Disease (AASLD) (10) and others (11), locoregional therapy, specifically TACE, is recommended as a first-line therapy for non-curative, nonsurgical management of patients with large multifocal HCC.
Prior studies have evaluated the efficacy and safety of locoregional therapy, with TACE having been shown to improve patient survival compared to supportive care alone in those with unresectable HCC (12,13); however, these studies have focused predominantly on the use of TACE and have not evaluated the possible benefit of using newer modalities such as radioembolization with yittrium-90. It has previously been established that LRT are effective in patients awaiting transplantation. This study aimed to determine features that are predictive of 3-year, intermediate-term survival in patients undergoing locoregional therapy for HCC as a destination therapeutic approach, focusing specifically on patients 60 years of age and older who are unwilling or unable to undergo surgical treatment.
Our results suggest that patient demographic factors do not have a significant impact on intermediate-term survival and that rather host liver function at diagnosis of HCC and immediately following locoregional therapy (represented by the MELD score) are more predictive. This appears to have been largely driven by an elevated INR in our cohort. This is in contrast to what was previously found in a study by Sato et al., which suggested that age greater than 70 was associated with significant mortality following treatment with locoregional therapy, specifically RFA (14). Two previous studies, however, found that advancing stage of HCC, not age of the patient, influenced patient survival when stratified by treatment subgroups (15,16). Our results echo these findings and additionally show that a wider range of treatment modalities may be applicable given that no significant difference was observed in terms of survival among the various locoregional techniques used.
Though this study included a large number of HCC patients over the age of 60 with a long-term follow-up period, and included a wide array of treatment modalities, it is not without limitations. First, a selection bias may be inherent given that data was pooled from a single center. In addition, results were based on initial treatment session despite many patients undergoing multiple treatment sessions and/or modalities. Prospective trials would be beneficial to further support our findings.
Destination therapy may be increasingly employed in the elderly population and our results suggest that locoregional approaches as “destination therapy” for patients with HCC presenting within transplant criteria who are either ineligible or unwilling result in modest intermediate-term survival (roughly 40%). Liver function at presentation and immediately following initiation of locoregional therapy, rather than demographics, tumor characteristics, or treatment modality are robust predictors of survival at 3 years. Further prospective studies are needed to assess optimization of candidate selection and modality-specific regimens with this approach.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the committee of Banner Health (#0004299) and waiver of informed consent was obtained for this retrospective study.
References
- Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer 2016;122:1312-37. [Crossref] [PubMed]
- Petrick JL, Kelly SP, Altekruse SF, et al. Future of Hepatocellular Carcinoma Incidence in the United States Forecast Through 2030. J Clin Oncol 2016;34:1787-94. [Crossref] [PubMed]
- Dhir M, Lyden ER, Smith LM, et al. Comparison of outcomes of transplantation and resection in patients with early hepatocellular carcinoma: a meta-analysis. HPB (Oxford) 2012;14:635-45. [Crossref] [PubMed]
- Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant:long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61:1968-77. [Crossref] [PubMed]
- Green TJ, Rochon PJ, Chang S, et al. Downstaging disease in patients with hepatocellular carcinoma outside of Milan Criteria: Strategies using drug-elutig bead chemoembolization. J Vasc Interv Radiol 2013;24:1613-22. [Crossref] [PubMed]
- Poggi G, Tosoratti N, Montagna B, et al. Microwave ablation of hepatocellular carcinoma. World J Hepatol 2015;7:2578-89. [Crossref] [PubMed]
- Mahnken AH, Bruners P, Günther RW. Local ablative therapies in HCC: Percutaneous ethanol injection and radiofrequency ablation. Dig Dis 2009;27:148-56. [Crossref] [PubMed]
- Cohen MJ, Bloom AI, Barak O, et al. Trans-arterial chemo-embolization is safe and effective for very elderly patients with hepatocellular carcinoma. World J Gastroenterol 2013;19:2521-8. [Crossref] [PubMed]
- Molla N, AlMenieir N, Simoneau E, et al. The role of interventional radiology in the management of hepatocellular carcinoma. Curr Oncol 2014;21:e480-92. [Crossref] [PubMed]
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma; an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of Liver consensus recommendations on hepatocellular carcinoma. Hepatol Int 2010;4:439-74. [Crossref] [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429-42. [Crossref] [PubMed]
- Sato M, Tateishi R, Yasunaga H, et al. Mortality and morbidity of hepatectomy, radiofrequency ablation and embolization for hepatocellular carcinoma: a national survey of 54,145 patients. J Gastroenterol 2012;47:1125-33. [Crossref] [PubMed]
- Dohmen K, Shirahama M, Shigematsu H, et al. Optimal treatment strategy for elderly patients with hepatocellular carcinoma. J Gastroenterol Hepatol 2004;19:859-65. [Crossref] [PubMed]
- Borzio M, Dionigi E, Parisi G, et al. Management of hepatocellular carcinoma in the elderly. World J Hepatol 2015;7:1521-9. [Crossref] [PubMed]


