Pretreatment albumin may aid in patient selection for intrahepatic Y-90 microsphere transarterial radioembolization (TARE) for malignancies of the liver
Introduction
Hepatic malignancy including primary hepatobiliary tumors and metastases to the liver are common, with an estimated 46,000 new cases of primary hepatobiliary tumors in 2015 (1). In addition, the liver is a frequent site of metastasis for many common malignancies including breast and colon (2,3). In many patients, particularly those with metastatic disease, definitive management with surgical resection or liver transplantation is not feasible as many patients are medically unfit for surgery and often have uncontrolled disease elsewhere. Patients not eligible for definitive surgical management may undergo systemic chemotherapy, but many also benefit from targeted approaches such as radiofrequency ablation, chemoembolization, cryoembolization, external beam radiation therapy, or trans-arterial radioembolization (TARE) with yttrium-90 (Y-90) microspheres (4,5). TARE with Y-90, a beta emitter, exerts a tumoricidal effect using a dual-pronged attack that relatively spares normal liver tissue by virtue of the dual blood supply of the liver. Malignancies tend to derive their blood supply primarily from the hepatic artery while normal liver tissue derives the majority of its blood supply from the portal vein. This causes implantation of Y-90 microspheres injected through the hepatic artery to implant preferentially within liver tumors. There they exert an embolic effect, depriving the tumor of its blood supply, but also deliver tumoricidal doses of radiation of greater than 100 Gy (6).
Two types of Y-90 microspheres are available. TheraSpheres® are a glass bead with Y-90 as an integral constituent of the glass. TheraSpheres® are approved for unresectable hepatocellular carcinoma (HCC) under the provision of a humanitarian device exemption. SIR-spheres® are a Y-90 tagged resin microsphere approved for hepatic metastases from colorectal cancer (CRC); other uses are off-label (7). There is convincing evidence supporting the use of Y-90 TARE for patients with unresectable hepatic malignancies. In heavily pretreated patients, Y-90 TARE can induce partial response or stable disease in a majority (8,9). In a phase III trial randomizing patients to Y-90 TARE plus hepatic artery chemotherapy versus hepatic artery chemotherapy alone, addition of Y-90 improved partial and complete response rates with a trend towards improved overall survival (OS) (10). In a small phase II trial, systemic chemotherapy plus Y-90 TARE was superior to chemotherapy alone with respect to disease response and time to progressive disease, and with no difference in quality of life (11). In the large phase III SIRFLOX study, 530 patients with metastatic CRC with liver metastases were treated with modified FOLFOX chemotherapy (plus or minus bevacizumab). Patients were randomized to receive chemotherapy alone versus chemotherapy with the addition of Y-90 TARE with the first cycle of chemotherapy. The addition of Y-90 TARE did not improve progression-free survival overall. However, the addition of Y-90 TARE did improve median progression-free survival in the liver by approximately eight months (12).
The most common complication of TARE is a post-embolic syndrome which includes fatigue, abdominal pain, nausea, and weight loss. Other more rare complications include hepatic dysfunction, GI toxicities such as ulcers or bleeding, and pneumonitis. Pneumonitis is avoided by measuring hepatopulmonary shunting to ensure it is less than 20% prior to treatment. Hepatopulmonary shunting greater than 20% is a contraindication to treating; shunting between 10–19% requires a decrease in the dose administered (13). GI toxicity is decreased by placing coils in the gastroduodenal artery and other extrahepatic vessels that branch off the hepatic arteries, prior to treatment, and the use of proton-pump inhibitors (PPIs) or H2-blockers after treatment (14). Despite measures taken to prevent GI toxicity, the incidence of gastroduodenal ulceration following TARE is estimated to be between 2.9% and 4.8% (15).
In light of the risk of toxicity in a population that generally has competing morbidities and a palliative rather than definitive intent, selection of patients most likely to derive benefit from TARE is of key importance. Indicators of prognosis are relatively under-reported in the literature on Y-90 microembolization. Salem et al. reported a series of 290 patients with HCC in which significantly increased survival was observed in patients who were Childs-Pugh A (17.2 months) versus B (7.7 months) (16). In a study of 214 patients with CRC metastases fewer prior lines of therapy and specifically no use of biological agents, higher performance status, lower disease burden, and albumin >3 g/dL all independently predicted survival (17).
We reviewed 114 patients treated with TARE at our institution from March 2006 when the program was initiated, through May 2014, to determine the safety and efficacy in our heterogeneous patient population, and to determine what patient factors predicted for survival.
Methods
Patient selection
This retrospective review was approved by the Institutional Review Board at the Medical University of South Carolina. All patients treated with Y-90 TARE at our institution from March of 2006, when the program was initiated, through May 2014 were included. Sixteen patients received more than one Y-90 treatment. For patients who received more than one treatment, OS was estimated from the time of first treatment.
Technical information
Prior to initiation of treatment, all patients underwent baseline screening with a complete metabolic profile, liver angiography with 99-m technetium-labeled microaggregated albumin scintigraphy to identify both aberrant vasculature to the liver and gastrointestinal tract as well as the percentage of pulmonary shunting. The total volume of the liver, each lobe volume, and the tumor burden was determined using preoperative CT or MRI scan. The body surface area method was used to determine the activity of Y-90 to be delivered (18). Microspheres were administered through a catheter placed typically into the right and/or left hepatic artery accessed via either the femoral or radial artery. Following treatment most patients were discharged home on a PPI and seen in follow up in one month to assess toxicity, then seen regularly to monitor disease burden and requirements of further therapy.
The primary purpose of this study was to identify patient factors that could be used to predict survival after administration of Y-90 TARE while assessing the safety and efficacy of the procedure in a heterogeneous patient population. Patient characteristics analyzed included histology, whether prior chemotherapy had been given, pretreatment bilirubin, and pretreatment albumin. Safety was assessed by reviewing patient records for rates of treatment related toxicity, which was scored according to the RTOG scale (19).
Statistics
Kaplan-Meier estimates of OS from date of first procedure are reported. Potential prognostic factors for OS were evaluated using log rank tests and Cox proportional hazards models. A significant difference was defined as P<0.05. Patients were censored at the date of last follow up.
Results
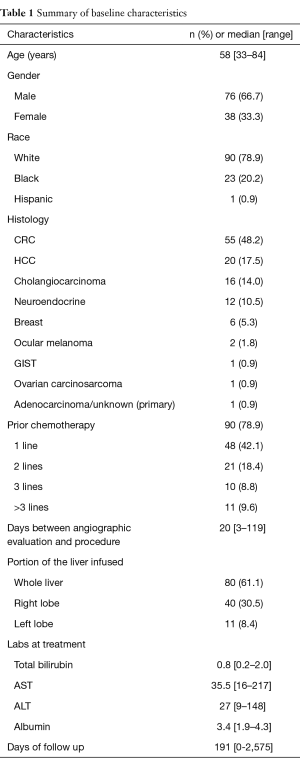
A total of 114 patients were treated with Y-90 TARE at our institution with a median follow up of 6.4 months (range 0–86 months). Table 1 lists the demographic characteristics at the time of initial treatment with TARE. The median age was 58 years (range, 33–84 years). The majority were males (66.7%); 78.9% were Caucasian, 20.2% were Black and 0.9% were Hispanic. The majority of patients (78.9%) had received at least one line of prior chemotherapy. Patients presented with a variety of histologies with metastatic CRC being the most common (48.2%), followed by HCC (17.5%), cholangiocarcinoma (CC) (14%), neuroendocrine (NE) (10.5%), and breast (BR) (5.3%). Other histologies treated included 2 patients with ocular melanoma and a single patient each with GIST, ovarian carcinosarcoma, and adenocarcinoma of unknown primary. The median baseline total bilirubin was 0.8 mg/dL (range, 0.2–2.0 mg/dL), median AST was 35.5 IU/L (range, 16–217 IU/L), median ALT was 27.0 IU/L (range, 9–148 IU/L), and median albumin was 3.4 g/dL (range, 1.9–4.3 g/dL). The majority of patients (61.1%), received treatment to the whole liver, with 30.5% receiving treatment to the right lobe only and 8.4% receiving treatment to only the left lobe.
Full table
The most commonly observed toxicity followed TARE was post embolization syndrome consisting of fatigue, nausea, low grade fever, and right upper quadrant pain. Grade 2 or greater toxicity was observed in 19.3% of patients following Y90 administration. Grade 2 toxicities included gastritis and ulcer formation, managed with a PPI and often with sucralfate. Grade 3 toxicity was observed in 7.9% of patients, consisting of melena or hematemesis from ulcer formation, managed with PPI and requiring transfusion in 5 patients. A single patient with a known pulmonary arteriovenous malformation developed pneumonitis which was managed with steroids. Grade 4 toxicity was seen in a single patient consisting of acute gangrenous cholecystitis successfully treated with a laparoscopic cholecystectomy; spheres were noted within the gallbladder on pathology. No deaths were attributed to radiation-induced liver disease.
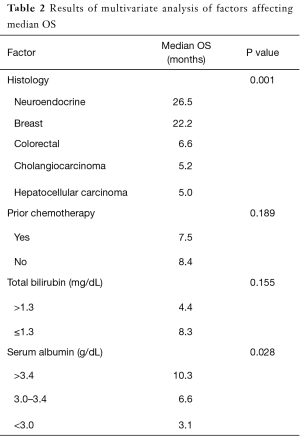
Median OS in the 114 patients treated with Y-90 TARE at out institution was 6.6 months, with a 6-month OS of 53.5% and 1-year OS of 30.7%. Patient factors including histology, prior treatment with chemotherapy, pretreatment albumin, and pretreatment total bilirubin were evaluated to determine whether predicted patient survival following administration of TARE (Table 2). Patients with NE and BR histologies had significantly better median OS (26.5 and 22.2 months respectively) than other histologies (P=0.001). A median OS of 6.6 months was observed in patients with a CR histology, 5.2 months median OS seen in patients with cholangiocarcinoma, and 5-month median OS in patients with HCC.
Full table
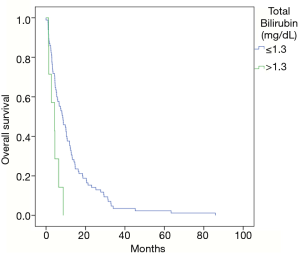
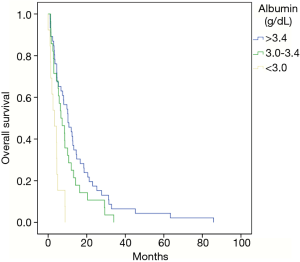
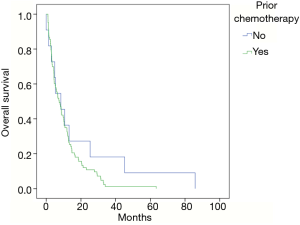
In patients with a non-hepatocellular histology, prior chemotherapy was given in 79% of patients with no significant difference in median OS observed between those receiving chemotherapy (7.4 months) and not receiving chemotherapy (8.4 months) (P=0.189) (Figure 1).The number of lines of chemotherapy administered prior to receiving TARE was also found to not significantly correlate with survival after treatment. No patient was treated with a total bilirubin greater than 2 mg/dL. Total bilirubin at the time of treatment was not found to correlate significantly with median OS. Patients with a total bilirubin greater than 1.3 mg/dL had a median OS of 4.4 months and those with a total bilirubin less than or equal to 1.3 mg/dL had a median OS of 8.3 months (P=0.115) (Figure 2). There was a statistically significant difference in median OS based on pretreatment serum albumin. With a normal serum albumin (greater than 3.4 at our institution) the median OS was observed to be 10.3 months, with an albumin between 3 and 3.4 g/dL median OS was 6.6 months, and with an albumin below 3 g/dL median OS was 3.1 months (P=0.028) (Figure 3).
Discussion
Our institutional experience with the use of Y-90 TARE for intrahepatic malignancies in 114 patients treated between March 2006 and May 2014 revealed it to be a relatively safe and effective treatment for intrahepatic malignancies. Our patients were heterogeneous, particularly in terms of demographics and histology which admittedly can make drawing conclusions difficult, however also represents a realistic representation of the patient population encountered in many clinics. The median age was 58 with a range of 33–84. The most common histology in our cohort was CRC which comprised 48.2% of our population. Median OS in this group was 6.6 months; previous series have reported a median OS range of 8.3–36 months (20). 62% of the CR patients in our series had two or more lines of chemotherapy (up to six) prior to TACE. We observed significantly better median OS in patients with NE and BR histologies, 26.5 and 22.2 months respectively. Previously reported median OS rates in BR have been reported between 10.8 and 20.9 months (21,22). In NE histologies median OS rates of 27.6 months have been reported (23). For NE cancers, 6-month OS rate was 53.5% and 12 months OS was 30.7% in our series. Previous reported data reports 6-month OS rates of 59–90% and 12-month OS rates of 0–39% (20).
We found no statistically significant difference in median OS based on whether chemotherapy had been given before TARE, though there was a trend toward improved survival in patients with non-hepatocellular histology who had not received chemotherapy prior to Y-90 TARE treatment. We also did not demonstrate a significant difference in median OS based on pretreatment total bilirubin, although all patients in this series had a total bilirubin of 2 mg/dL or less at the time of TARE. In patients with a normal total bilirubin (1.3 mg/dL or less) there was a trend toward improvement in median OS but it did not reach significance, possibly due to having only seven patients with a total bilirubin greater than 1.3 mg/dL at the time of treatment.
We did demonstrate a significant difference in median OS based on serum albumin at the time of treatment. We found a significant decline in OS as serum albumin, and thus hepatic synthetic function, decreased, even with the majority of patients having a normal total bilirubin and none having a total bilirubin greater than 2 mg/dL. Patients with a normal serum albumin had a greater median OS than that of our overall patient population (10.3 months compared to 6.6 months) and when albumin fell below 3 g/dL median OS fell to just 3.1 months, suggesting that patients with an albumin below 3 g/dL may not derive a significant clinical benefit from treatment with Y-90 TARE. An albumin >3 g/dL has previously been reported as a predictor of survival in a group of patients with colorectal histology treated with Y-90 TARE, however median OS based on albumin was not reported (17). We also found a statistically significant decrease in median OS in patients with a serum albumin below normal but above 3 g/dL.
In our experience Y-90 TARE was generally well tolerated, with the majority of patients experiencing only a mild post-embolization syndrome consisting of fatigue, nausea, low grade fever, and right upper quadrant pain, which has been reported to have an incidence of 20–70% (24). Grade 2 or greater toxicity was observed in 19.3% mostly consisting of gastritis and gastric or duodenal ulcers. The incidence of gastroduodenal ulceration following TARE is estimated to be between 2.9% and 4.8% (15). GI complications are secondary to hepaticoenteric vascular communications resulting microsphere deposition in the gastrointestinal tract. Prophylactic coiling of these communicating vessels is used to limit potential GI toxicity, though collateral vessels can form, requiring reassessment in patients undergoing repeat treatments (24). Prophylactic PPIs were typically used in this series. Another risk factor for GI toxicity is stasis during injection leading to reflux of microspheres (24). We report a grade 3 toxicity rate of 7.9% with either melena or hematemesis, mostly secondary to ulcer formation. Other large series have reported grade 3 toxicity rates of around 6–9% (24,25). A single patient developed acute gangrenous cholecystitis which was successfully managed with a laparoscopic cholecystectomy, with microspheres noted in the pathology specimen. This is a rare complication that has been previously reported (26). One method of prevention is to identify and coil the cystic artery. No deaths were attributed to radiation-induced liver disease, which has a reported incidence of up to 4%. Prior EBRT (External Beam Radiation Therapy) to the liver is a reported risk factor for developing RILD (Radiation Induced Liver Disease) (24). No patients treated on this series had previously received EBRT to the liver. One patient with a known pulmonary AVM developed RTOG grade 3 pneumonitis managed with steroids. Radiation pneumonitis is rare if patients are assessed for hepatopulmonary shunting prior to treatment. Hepatopulmonary shunting greater than 20% is a contraindication to treatment with Y90 TARE; shunting between 10–20% requires a decrease in the dose administered to ensure that the delivery to the lungs is not greater than 30 Gy (13).
Conclusions
Our experience with Y-90 TARE indicates it is a relatively safe and effective treatment option in properly screened patients with intrahepatic malignancies. Our data suggests that patients with NE and breast histologies have significantly better median OS than other histologies. In those patients with non-HCC histologies, we found that hepatic synthetic function as demonstrated by albumin, predicted for OS, despite all patients having a total bilirubin not greater than 2 mg/dL. Patients with a serum albumin below 3 g/dL may not derive significant clinical benefit from Y-90 TARE based on this series.
Acknowledgements
None.
Footnote
Conflicts of Interest: Data presented at ASCO GI Symposium 2015.
Ethical Statement: This retrospective review was approved by the Institutional Review Board at the Medical University of South Carolina (No. 0000020).
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Simmonds PC, Primrose JN, Colquitt JL, et al. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer 2006;94:982-99. [Crossref] [PubMed]
- Mayo SC, Pawlik TM. Current management of colorectal hepatic metastasis. Expert Rev Gastroenterol Hepatol 2009;3:131-44. [Crossref] [PubMed]
- Livraghi T, Makisalo H, Line PD. Treatment options in hepatocellular carcinoma today. Scand J Surg 2011;100:22-9. [Crossref] [PubMed]
- Vente MA, Hobbelink MG, van Het Schip AD, et al. Radionuclide liver cancer therapies: from concept to current clinical status. Anticancer Agents Med Chem 2007;7:441-59. [Crossref] [PubMed]
- Murthy R, Kamat P, Nunez R, et al. Radioembolization of yttrium-90 microspheres for hepatic malignancy. Semin Intervent Radiol 2008;25:48-57. [Crossref] [PubMed]
- Vente MA, Wondergem M, van der Tweel I, et al. Yttrium-90 microsphere radioembolization for the treatment of liver malignancies: a structured meta-analysis. Eur Radiol 2009;19:951-9. [Crossref] [PubMed]
- Cosimelli M, Golfieri R, Cagol PP, et al. Multi-centre phase II clinical trial of yttrium-90 resin microspheres alone in unresectable, chemotherapy refractory colorectal liver metastases. Br J Cancer 2010;103:324-31. [Crossref] [PubMed]
- Mancini R, Carpanese L, Sciuto R, et al. A multicentric phase II clinical trial on intra-arterial hepatic radiotherapy with 90yttrium SIR-spheres in unresectable, colorectal liver metastases refractory to i.v. chemotherapy: preliminary results on toxicity and response rates. In Vivo 2006;20:711-4. [PubMed]
- Gray B, Van Hazel G, Hope M, et al. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol 2001;12:1711-20. [Crossref] [PubMed]
- Van Hazel G, Blackwell A, Anderson J, et al. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol 2004;88:78-85. [Crossref] [PubMed]
- van Hazel GA, Heinemann V, Sharma NK, et al. SIRFLOX: Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients With Metastatic Colorectal Cancer. J Clin Oncol 2016;34:1723-31. [Crossref] [PubMed]
- Hilgard P, Hamami M, Fouly AE, et al. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology 2010;52:1741-9. [Crossref] [PubMed]
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. [Crossref] [PubMed]
- Naymagon S, Warner RR, Patel K, et al. Gastroduodenal ulceration associated with radioembolization for the treatment of hepatic tumors: an institutional experience and review of the literature. Dig Dis Sci 2010;55:2450-8. [Crossref] [PubMed]
- Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology 2010;138:52-64. [Crossref] [PubMed]
- Lewandowski RJ, Memon K, Mulcahy MF, et al. Twelve-year experience of radioembolization for colorectal hepatic metastases in 214 patients: survival by era and chemotherapy. Eur J Nucl Med Mol Imaging 2014;41:1861-9. [Crossref] [PubMed]
- Mosteller RD. Simplified Calculation of Body Surface Area. N Engl J Med 1987;317:1098. [Crossref] [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341-6. [Crossref] [PubMed]
- Saxena A, Bester L, Shan L, et al. A systemic review on the safety and efficacy of yttrium-90 radioembolization for unresectable, chemorefractory colorectal cancer liver metastases. J Cancer Res Clin Oncol 2014;140:537-47. [Crossref] [PubMed]
- Saxena A, Kapoor J, Meteling B, et al. Yttrium-90 radioembolization for unresectable, chemoresistant breast cancer liver metastases: a large single-center experience of 40 patients. Ann Surg Oncol 2014;21:1296-303. [Crossref] [PubMed]
- Smits ML, Prince JF, Rosenbaum CE, et al. Intra-arterial radioembolization of breast cancer liver metastases: a structured review. Eur J Pharmacol 2013;709:37-42. [Crossref] [PubMed]
- King J, Quinn R, Glenn DM, et al. Radioembolization with selective internal radiation microspheres for neuroendocrine liver metastases. Cancer 2008;113:921-9. [Crossref] [PubMed]
- Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol 2014;4:198. [Crossref] [PubMed]
- Kennedy A. Radioembolization of hepatic tumors. J Gastrointest Oncol 2014;5:178-9. [PubMed]
- Atassi B, Bangash AK, Lewandowski RJ, et al. Biliary sequelae following radioembolization with Y90 microspheres. JVIR 2008;19:691-7. [Crossref] [PubMed]